Glutamate ionotropic receptor kainate type subunit 2, also known as ionotropic glutamate receptor 6 or GluR6, is a protein that in humans is encoded by the GRIK2 (or GLUR6) gene. [5] [6] [7]
Glutamate ionotropic receptor kainate type subunit 2, also known as ionotropic glutamate receptor 6 or GluR6, is a protein that in humans is encoded by the GRIK2 (or GLUR6) gene. [5] [6] [7]
This gene encodes a subunit of a kainate glutamate receptor. This receptor may have a role in synaptic plasticity, learning, and memory. It also may be involved in the transmission of visual information from the retina to the hypothalamus. The structure and function of the encoded protein is influenced by RNA editing. Alternatively spliced transcript variants encoding distinct isoforms have been described for this gene. [7] It has been discovered that this is a key protein, which enables mammals to feel cold sensations. [8]
Homozygosity for a GRIK2 deletion-inversion mutation is associated with non-syndromic autosomal recessive mental retardation. [9]
GRIK2 has been shown to interact with:
Pre-mRNA for several neurotransmitter receptors and ion channels are substrates for ADARs, including AMPA receptor subunits (GluR2, GluR3, GluR4) and kainate receptor subunits (GluR5, GluR6). Glutamate-gated ion channels are made up of four subunits per channel, with each subunit contributing to the pore loop structure. The pore loop structure is similar to that found in K+ channels (e.g. the human Kv1.1 channel, whose pre-mRNA is also subject to A to I RNA editing). [17] [18] The diversity of ionotropic glutamate receptor subunits, as well as RNA splicing, is determined by RNA editing events of the individual subunits, explaining their extremely high diversity.
The type of RNA editing that occurs in the pre-mRNA of GluR6 is Adenosine to Inosine (A to I) editing. [19]
A to I RNA editing is catalyzed by a family of adenosine deaminases acting on RNA (ADARs) that specifically recognize adenosines within double-stranded regions of pre-mRNAs and deaminate them to inosine. Inosines are recognised as guanosine by the cell's translational machinery. There are three members of the ADAR family ADARs 1–3 with ADAR1 and ADAR2 being the only enzymatically active members. ADAR1 and ADAR2 are widely expressed in tissues, while ADAR3 is restricted to the brain, where it is though tot have a regulatory role. The double-stranded regions of RNA are formed by base-pairing between residues close to region of the editing site, with residues usually in a neighboring intron, though they can occasionally be located in an exonic sequence. The region that forms base pairs with the editing region is known as an Editing Complementary Sequence (ECS).
ADARs bind interact directly with the dsRNA substrate via their double-stranded RNA binding domains. If an editing site occurs within a coding sequence, the result could be a codon change. This can lead to translation of a protein isoform due to a change in its primary protein structure. Therefore, editing can also alter protein function. A to I editing occurs in a noncoding RNA sequences such as introns, untranslated regions (UTRs), LINEs, and SINEs (especially Alu repeats). The function of A to I editing in these regions is thought to involve creation of splice sites and retention of RNAs in the nucleus amongst others.
The pre-mRNA of GLUR6 is edited at amino acid positions 567, 571, and 621. The Q/R position, which gets its name as editing results in an codon change from a glutamine (Q) codon (CAG) to an arginine (R) codon (CGG), is located in the "pore loop" of the second membrane domain (M2). The Q/R site of GluR6 pre-mRNA occurs in an asymmetrical loop of three exonic and four intronic nucleotides. The Q/R editing site is also observed in GluR2 and GluR5. The Q/R site is located in a homologous position in GluR2 and in GluR6. [20]
GluR-6 is also edited at I/V and Y/C sites, which are found in the first membrane domain (M1). At the I/V site, editing results in a codon change from (ATT) isoleucine (I) to (GTT) valine (V), while at the Y/C site, the codon change is from (TAC) tyrosine (Y) to (TGC) cysteine (C). [21]
The RNAfold program characterised a putative double-stranded RNA (dsRNA) conformation around the Q/R site of the GluR-6 pre-mRNA. This sequence is necessary for editing at the site to occur. The possible editing complementary sequence was observed from transcript analysis to be 1.9 kb downstream from the editing site within intron 12. [20] The ECS for the editing sites in M1 has yet to be identified but it is likely to occur at a considerable distance from the editing sites. [22]
Editing of the Q/R site in GluR6 pre-mRNA has been demonstrated to be developmentally regulated in rats, ranging from 0% in rat embryo to 80% at birth. This is different from the AMPA receptor subunit GluR2, which is nearly 100% edited and is not developmentally regulated. [21] Significant amounts of both edited and non-edited forms of GluR6 transcripts are found in the adult brain. The receptor is 90% edited in all grey matter structures, while in white matter, the receptor is edited in just 10% of cases. Frequency increases from 0% in rat embryo to 85% in adult rat.
The primary GluR6 transcripts can be edited in up to three positions. Editing at each of the three positions affects Ca2+ permeability of the channel. [23]
Editing plays a role in the electrophysiology of the channel. Editing at the Q/R site has been deemed to be nonessential in GluR6. [24] It has been reported that the unedited version of GluR6 functions in the regulation of synaptic plasticity. The edited version is thought to inhibit synaptic plasticity and reduce seizure susceptibility. [23] Mice lacking the Q/R site exhibit increased long term potentiation and are more susceptible to kainate induced seizures. The number of seizures is inversely correlated with the amount of RNA editing. Human GluR6 pre-mRNA editing is increased during seizures, possibly as an adaptive mechanism. [25] [26]
Up to 8 different protein isoforms can occur as a result of different combinations of editing at the three sites, giving rise to receptor variants with differing kinetics. The effect of Q/R site editing on calcium permeability appears to be dependent on editing of the I/V and Y/C sites. When both sites in TM1 (I/V and Y/C) are edited, Q/R site editing is required for calcium permeability. On the contrary, when neither the I/V nor the Y/C site is edited, receptors demonstrate high calcium permeability regardless of Q/R site editing. The co-assembly of these two isoforms generate receptors with reduced calcium permeability. [23]
RNA editing of the Q/R site can affect inhibition of the channel by membrane fatty acids such as arachidonic acid and docosahexaenoic acid [27] For Kainate receptors with only edited isforms, these are strongly inhibited by these fatty acids, however inclusion of just one non-edited subunit is enough to abolish this effect. [27]
Kainate-induced seizures in mice are used as a model of temporal lobe epilepsy in humans. Despite mice deficient in editing at the Q/R site of GluR6 showing increased seizure susceptibility, tissue analysis of human epilepsy patients did not show reduced editing at this site. [24] [28] [29] [30]

The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic transmembrane receptor for glutamate (iGluR) and predominantly Na+ ion channel that mediates fast synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The GRIA2-encoded AMPA receptor ligand binding core (GluA2 LBD) was the first glutamate receptor ion channel domain to be crystallized.

Kainate receptors, or kainic acid receptors (KARs), are ionotropic receptors that respond to the neurotransmitter glutamate. They were first identified as a distinct receptor type through their selective activation by the agonist kainate, a drug first isolated from the algae Digenea simplex. They have been traditionally classified as a non-NMDA-type receptor, along with the AMPA receptor. KARs are less understood than AMPA and NMDA receptors, the other ionotropic glutamate receptors. Postsynaptic kainate receptors are involved in excitatory neurotransmission. Presynaptic kainate receptors have been implicated in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA through a presynaptic mechanism.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.
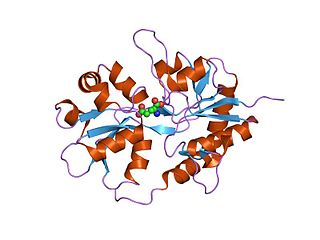
Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels that are activated by the neurotransmitter glutamate. They mediate the majority of excitatory synaptic transmission throughout the central nervous system and are key players in synaptic plasticity, which is important for learning and memory. iGluRs have been divided into four subtypes on the basis of their ligand binding properties (pharmacology) and sequence similarity: AMPA receptors, kainate receptors, NMDA receptors and delta receptors.

Glutamate receptor 3 is a protein that in humans is encoded by the GRIA3 gene.

Filamin A, alpha (FLNA) is a protein that in humans is encoded by the FLNA gene.

Glutamate receptor, metabotropic 6, also known as GRM6 or mGluR6, is a protein which in humans is encoded by the GRM6 gene.

Glutamate [NMDA] receptor subunit epsilon-1 is a protein that in humans is encoded by the GRIN2A gene. With 1464 amino acids, the canonical GluN2A subunit isoform is large. GluN2A-short isoforms specific to primates can be produced by alternative splicing and contain 1281 amino acids.
Glutamate [NMDA] receptor subunit zeta-1 is a protein that in humans is encoded by the GRIN1 gene.

Glutamate receptor 1 is a protein that in humans is encoded by the GRIA1 gene.

Glutamate ionotropic receptor AMPA type subunit 2 is a protein that in humans is encoded by the GRIA2 gene and it is a subunit found in the AMPA receptors.

Glutamate receptor, ionotropic, kainate 1, also known as GRIK1, is a protein that in humans is encoded by the GRIK1 gene.

Glutamate receptor 4 is a protein that in humans is encoded by the GRIA4 gene.

Glutamate receptor, ionotropic, delta 2, also known as GluD2, GluRδ2, or δ2, is a protein that in humans is encoded by the GRID2 gene. This protein together with GluD1 belongs to the delta receptor subtype of ionotropic glutamate receptors. They possess 14–24% sequence homology with AMPA, kainate, and NMDA subunits, but, despite their name, do not actually bind glutamate or various other glutamate agonists.

Glutamate receptor, ionotropic kainate 3 is a protein that in humans is encoded by the GRIK3 gene.

Glutamate receptor, ionotropic kainate 5 is a protein that in humans is encoded by the GRIK5 gene.

Glutamate receptor delta-1 subunit also known as GluD1 or GluRδ1 is a transmembrane protein encoded by the GRID1 gene. A C-terminal GluD1 splicing isoform has been described based on mRNA analysis.

GRIK4 is a kainate receptor subtype belonging to the family of ligand-gated ion channels which is encoded by the GRIK4 gene.
Within the science of molecular biology and cell biology, for human genetics, the GRIA2 gene is located on chromosome 4q32-q33. The gene product is the ionotropic AMPA glutamate receptor 2. The protein belongs to a family of ligand-activated glutamate receptors that are sensitive to alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA). Glutamate receptors function as the main excitatory neurotransmitter at many synapses in the central nervous system. L-glutamate, an excitatory neurotransmitter, binds to the Gria2 resulting in a conformational change. This leads to the opening of the channel converting the chemical signal to an electrical impulse. AMPA receptors (AMPAR) are composed of four subunits, designated as GluR1 (GRIA1), GluR2 (GRIA2), GluR3 (GRIA3), and GluR4(GRIA4) which combine to form tetramers. They are usually heterotrimeric but can be homodimeric. Each AMPAR has four sites to which an agonist can bind, one for each subunit.[5]

Willardiine (correctly spelled with two successive i's) or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids (See:#Synthesis). Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana (another name for Mariosousa willardiana) when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.