Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.

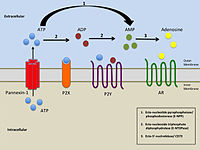
Purinergic receptors, also known as purinoceptors, are a family of plasma membrane molecules that are found in almost all mammalian tissues. Within the field of purinergic signalling, these receptors have been implicated in learning and memory, locomotor and feeding behavior, and sleep. More specifically, they are involved in several cellular functions, including proliferation and migration of neural stem cells, vascular reactivity, apoptosis and cytokine secretion. These functions have not been well characterized and the effect of the extracellular microenvironment on their function is also poorly understood.

The P2X receptors, also ATP-gated P2X receptor cation channel family, is a protein family that consists of cation-permeable ligand-gated ion channels that open in response to the binding of extracellular adenosine 5'-triphosphate (ATP). They belong to a larger family of receptors known as the ENaC/P2X superfamily. ENaC and P2X receptors have similar 3-D structures and are homologous. P2X receptors are present in a diverse array of organisms including humans, mouse, rat, rabbit, chicken, zebrafish, bullfrog, fluke, and amoeba.

P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14.

Toll-like receptor 4 (TLR4), also designated as CD284, is a key activator of the innate immune response and plays a central role in the fight against bacterial infections. TLR4 is a transmembrane protein of approximately 95 kDa that is encoded by the TLR4 gene.
Gliotransmitters are chemicals released from glial cells that facilitate neuronal communication between neurons and other glial cells. They are usually induced from Ca2+ signaling, although recent research has questioned the role of Ca2+ in gliotransmitters and may require a revision of the relevance of gliotransmitters in neuronal signalling in general.

P2Y purinoceptor 1 is a protein that in humans is encoded by the P2RY1 gene.

P2X purinoceptor 1, also ATP receptor, is a protein that in humans is encoded by the P2RX1 gene.

P2Y purinoceptor 2 is a protein that in humans is encoded by the P2RY2 gene.

Ectonucleoside triphosphate diphosphohydrolase-1 also known as CD39, is a typical cell surface enzyme with a catalytic site on the extracellular face.

P2Y purinoceptor 11 is a protein that in humans is encoded by the P2RY11 gene.

P2X purinoceptor 4 is a protein that in humans is encoded by the P2RX4 gene. P2X purinoceptor 4 is a member of the P2X receptor family. P2X receptors are trimeric protein complexes that can be homomeric or heteromeric. These receptors are ligand-gated cation channels that open in response to ATP binding. Each receptor subtype, determined by the subunit composition, varies in its affinity to ATP and desensitization kinetics.

P2X purinoceptor 5 is a protein that in humans is encoded by the P2RX5 gene.
P2X purinoceptor 3 is a protein that in humans is encoded by the P2RX3 gene.

P2X purinoceptor 6 is a protein that in humans is encoded by the P2RX6 gene.
Damage-associated molecular patterns (DAMPs) are molecules within cells that are a component of the innate immune response released from damaged or dying cells due to trauma or an infection by a pathogen. They are also known as danger signals, and alarmins because they serve as warning signs to alert the organism to any damage or infection to its cells. DAMPs are endogenous danger signals that are discharged to the extracellular space in response to damage to the cell from mechanical trauma or a pathogen. Once a DAMP is released from the cell, it promotes a noninfectious inflammatory response by binding to a pattern recognition receptor. Inflammation is a key aspect of the innate immune response; it is used to help mitigate future damage to the organism by removing harmful invaders from the affected area and start the healing process. As an example, the cytokine IL-1α is a DAMP that originates within the nucleus of the cell which, once released to the extracellular space, binds to the PRR IL-1R, which in turn initiates an inflammatory response to the trauma or pathogen that initiated the release of IL-1α. In contrast to the noninfectious inflammatory response produced by DAMPs, pathogen-associated molecular patterns initiate and perpetuate the infectious pathogen-induced inflammatory response. Many DAMPs are nuclear or cytosolic proteins with defined intracellular function that are released outside the cell following tissue injury. This displacement from the intracellular space to the extracellular space moves the DAMPs from a reducing to an oxidizing environment, causing their functional denaturation, resulting in their loss of function. Outside of the aforementioned nuclear and cytosolic DAMPs, there are other DAMPs originated from different sources, such as mitochondria, granules, the extracellular matrix, the endoplasmic reticulum, and the plasma membrane.
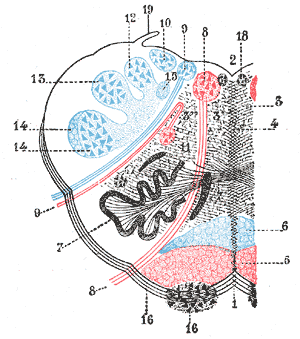
The rostral ventromedial medulla (RVM), or ventromedial nucleus of the spinal cord, is a group of neurons located close to the midline on the floor of the medulla oblongata. The rostral ventromedial medulla sends descending inhibitory and excitatory fibers to the dorsal horn spinal cord neurons. There are 3 categories of neurons in the RVM: on-cells, off-cells, and neutral cells. They are characterized by their response to nociceptive input. Off-cells show a transitory decrease in firing rate right before a nociceptive reflex, and are theorized to be inhibitory. Activation of off-cells, either by morphine or by any other means, results in antinociception. On-cells show a burst of activity immediately preceding nociceptive input, and are theorized to be contributing to the excitatory drive. Neutral cells show no response to nociceptive input.

Purinergic signalling is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.
The prostaglandin D2 (PGD2) receptors are G protein-coupled receptors that bind and are activated by prostaglandin D2. Also known as PTGDR or DP receptors, they are important for various functions of the nervous system and inflammation. They include the following proteins:
Microglia are the primary immune cells of the central nervous system, similar to peripheral macrophages. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells.