
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.

Ilmenite is a titanium-iron oxide mineral with the idealized formula FeTiO
3. It is a weakly magnetic black or steel-gray solid. Ilmenite is the most important ore of titanium and the main source of titanium dioxide, which is used in paints, printing inks, fabrics, plastics, paper, sunscreen, food and cosmetics.

Baddeleyite is a rare zirconium oxide mineral (ZrO2 or zirconia), occurring in a variety of monoclinic prismatic crystal forms. It is transparent to translucent, has high indices of refraction, and ranges from colorless to yellow, green, and dark brown. See etymology below.

Anorthosite is a phaneritic, intrusive igneous rock characterized by its composition: mostly plagioclase feldspar (90–100%), with a minimal mafic component (0–10%). Pyroxene, ilmenite, magnetite, and olivine are the mafic minerals most commonly present.

Armalcolite is a titanium-rich mineral with the chemical formula (Mg,Fe2+)Ti2O5. It was first found at Tranquility Base on the Moon in 1969 during the Apollo 11 mission, and is named for Armstrong, Aldrin and Collins, the three Apollo 11 astronauts. Together with tranquillityite and pyroxferroite, it is one of three new minerals that were discovered on the Moon. Armalcolite was later identified at various locations on Earth and has been synthesized in the laboratory. (Tranquillityite and pyroxferroite were also later found at various locations on Earth). The synthesis requires low pressures, high temperatures and rapid quenching from about 1,000 °C to the ambient temperature. Armalcolite breaks down to a mixture of magnesium-rich ilmenite and rutile at temperatures below 1,000 °C, but the conversion slows down with cooling. Because of this quenching requirement, armalcolite is relatively rare and is usually found in association with ilmenite and rutile, among other minerals.

Coesite is a form (polymorph) of silicon dioxide (SiO2) that is formed when very high pressure (2–3 gigapascals), and moderately high temperature (700 °C, 1,300 °F), are applied to quartz. Coesite was first synthesized by Loring Coes, Jr., a chemist at the Norton Company, in 1953.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Komatiite is a type of ultramafic mantle-derived volcanic rock defined as having crystallised from a lava of at least 18 wt% magnesium oxide (MgO). It is classified as a 'picritic rock'. Komatiites have low silicon, potassium and aluminium, and high to extremely high magnesium content. Komatiite was named for its type locality along the Komati River in South Africa, and frequently displays spinifex texture composed of large dendritic plates of olivine and pyroxene.

Chondrodite is a nesosilicate mineral with formula (Mg,Fe)
5(SiO
4)
2(F,OH,O)
2. Although it is a fairly rare mineral, it is the most frequently encountered member of the humite group of minerals. It is formed in hydrothermal deposits from locally metamorphosed dolomite. It is also found associated with skarn and serpentinite. It was discovered in 1817 at Pargas in Finland, and named from the Greek for "granule", which is a common habit for this mineral.

Pyroxferroite (Fe2+,Ca)SiO3 is a single chain inosilicate. It is mostly composed of iron, silicon and oxygen, with smaller fractions of calcium and several other metals. Together with armalcolite and tranquillityite, it is one of the three minerals which were discovered on the Moon during the 1969 Apollo 11 mission. It was then found in Lunar and Martian meteorites as well as a mineral in the Earth's crust. Pyroxferroite can also be produced by annealing synthetic clinopyroxene at high pressures and temperatures. The mineral is metastable and gradually decomposes at ambient conditions, but this process can take billions of years.
In geology ultrahigh-temperature metamorphism (UHT) is extreme crustal metamorphism with metamorphic temperatures exceeding 900 °C. Granulite-facies rocks metamorphosed at very high temperatures were identified in the early 1980s, although it took another decade for the geoscience community to recognize UHT metamorphism as a common regional phenomenon. Petrological evidence based on characteristic mineral assemblages backed by experimental and thermodynamic relations demonstrated that Earth's crust can attain and withstand very high temperatures (900–1000 °C) with or without partial melting.
Akimotoite is a rare silicate mineral in the ilmenite group of minerals, with the chemical formula (Mg,Fe)SiO3. It is polymorphous with pyroxene and with bridgmanite, a natural silicate perovskite that is the most abundant mineral in Earth's silicate mantle. Akimotoite has a vitreous luster, is colorless, and has a white or colorless streak. It crystallizes in the trigonal crystal system in space group R3. It is the silicon analogue of geikielite (MgTiO3).

A melt inclusion is a small parcel or "blobs" of melt(s) that is entrapped by crystals growing in magma and eventually forming igneous rocks. In many respects it is analogous to a fluid inclusion within magmatic hydrothermal systems. Melt inclusions tend to be microscopic in size and can be analyzed for volatile contents that are used to interpret trapping pressures of the melt at depth.
A whiteschist is an uncommon metamorphic rock formed at high to ultra-high pressures. It has the characteristic mineral assemblage of kyanite + talc, responsible for its white colour. The name was introduced in 1973 by German mineralogist and petrologist Werner Schreyer. This rock is associated with the metamorphism of some pelites, evaporite sequences or altered basaltic or felsic intrusions. Whiteschists form in the MgO–Fe
2O
3–Al
2O
3–SiO
2–H
2O (MFASH) system. Rocks of this primary chemistry are extremely uncommon and they are in most cases thought to be the result of metasomatic alteration, with the removal of various mobile elements.
Silicate perovskite is either (Mg,Fe)SiO3 or CaSiO3 when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km depth. They are thought to form the main mineral phases, together with ferropericlase.

Pyrophanite is a manganese titanium oxide mineral with formula: MnTiO3. It is a member of the ilmenite group. It is a deep red to greenish black mineral which crystallizes in the trigonal system.

Timothy John Barrington Holland is a petrologist and Emeritus Professor in the Department of Earth Sciences at the University of Cambridge.

Titanium in zircon geothermometry is a form of a geothermometry technique by which the crystallization temperature of a zircon crystal can be estimated by the amount of titanium atoms which can only be found in the crystal lattice. In zircon crystals, titanium is commonly incorporated, replacing similarly charged zirconium and silicon atoms. This process is relatively unaffected by pressure and highly temperature dependent, with the amount of titanium incorporated rising exponentially with temperature, making this an accurate geothermometry method. This measurement of titanium in zircons can be used to estimate the cooling temperatures of the crystal and infer conditions during which it crystallized. Compositional changes in the crystals growth rings can be used to estimate the thermodynamic history of the entire crystal. This method is useful as it can be combined with radiometric dating techniques that are commonly used with zircon crystals, to correlate quantitative temperature measurements with specific absolute ages. This technique can be used to estimate early Earth conditions, determine metamorphic facies, or to determine the source of detrital zircons, among other uses.

Mark S. Ghiorso is an American geochemist who resides in Seattle, Washington. He is best known for creating MELTS, a software tool for thermodynamic modeling of phase equilibria in magmatic systems.
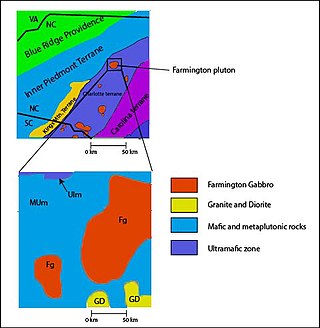
Located in the Charlotte Belt of North Carolina is the Farmington Gabbro, located in the Mocksville Complex. The Mocksville Complex consist of metamorphosed/unmetamorphosed gabbros, pyroxenites, hornblendites, wehrlites, granites, and diorites. The plutons in this region formed during the Taconic, Acadian, and Alleghanian orogeny starting on the eastern side of Laurentia. These plutons date back to around 400 Ma, consisting of ultramafic, mafic, and felsic rocks but the Farmington Gabbro is the only pluton on the northwest side of the complex that is unmetamorphosed.
















