In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

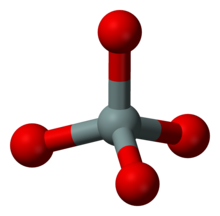
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula [SiO(4-2x)−
4−x]
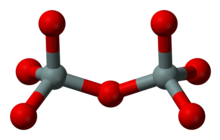
n, where 0 ≤ x < 2. The family includes orthosilicate SiO4−4, metasilicate SiO2−3, and pyrosilicate Si2O6−7. The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate [SiF6]2−.Most commonly, silicates are encountered as silicate minerals.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron. Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

The Borate Minerals are minerals which contain a borate anion group. The borate (BO3) units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures which include hydroxide or halogen anions. The [B(O,OH)4]− anion exists as well.

Phosphate minerals contain the tetrahedrally coordinated phosphate (PO43−) anion, sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions, along with chloride (Cl−), fluoride (F−), and hydroxide (OH−) anions, that also fit into the crystal structure.

Carbonate minerals are those minerals containing the carbonate ion, CO2−
3.
Arsenate minerals usually refer to the naturally occurring orthoarsenates, possessing the (AsO4)3− anion group and, more rarely, other arsenates with anions like AsO3(OH)2− (also written HAsO42−) (example: pharmacolite Ca(AsO3OH).2H2O) or (very rarely) [AsO2(OH)2]− (example: andyrobertsite). Arsenite minerals are much less common. Both the Dana and the Strunz mineral classifications place the arsenates in with the phosphate minerals.

The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. Minerals with complex anion groups such as the silicates, sulfates, carbonates and phosphates are classed separately.

Hauyne or haüyne, also called hauynite or haüynite, is a tectosilicate sulfate mineral with endmember formula Na3Ca(Si3Al3)O12(SO4). As much as 5 wt % K2O may be present, and also H2O and Cl. It is a feldspathoid and a member of the sodalite group. Hauyne was first described in 1807 from samples discovered in Vesuvian lavas in Monte Somma, Italy, and was named in 1807 by Brunn-Neergard for the French crystallographer René Just Haüy (1743–1822). It is sometimes used as a gemstone.

Clinozoisite is a complex calcium aluminium sorosilicate mineral with formula: Ca2Al3(Si2O7)(SiO4)O(OH). It forms a continuous solid solution series with epidote by substitution of iron(III) in the aluminium (m3 site) and is also called aluminium epidote.

Melilite refers to a mineral of the melilite group. Minerals of the group are solid solutions of several endmembers, the most important of which are gehlenite and åkermanite. A generalized formula for common melilite is (Ca,Na)2(Al,Mg,Fe2+)[(Al,Si)SiO7]. Discovered in 1793 near Rome, it has a yellowish, greenish-brown color. The name derives from the Greek words meli (μέλι) "honey" and lithos (λίθους) "stone".The name refers to a group of minerals (melilite group) with chemically similar composition, nearly always minerals in åkermanite-gehlenite series.
In inorganic chemistry, mineral hydration is a reaction which adds water to the crystal structure of a mineral, usually creating a new mineral, commonly called a hydrate.

Julgoldite is a member of the pumpellyite mineral series, a series of minerals characterized by the chemical bonding of silica tetrahedra with alkali and transition metal cations. Julgoldites, along with more common minerals like epidote and vesuvianite, belong to the subclass of sorosilicates, the rock-forming minerals that contain SiO4 tetrahedra that share a common oxygen to form Si2O7 ions with a charge of 6− (Deer et al., 1996). Julgoldite has been recognized for its importance in low grade metamorphism, forming under shear stress accompanied by relatively low temperatures (Coombs, 1953). Julgoldite was named in honor of Professor Julian Royce Goldsmith (1918–1999) of the University of Chicago.
The mineralogy of Mars is the chemical composition of rocks and soil that encompass the surface of Mars. Various orbital crafts have used spectroscopic methods to identify the signature of some minerals. The planetary landers performed concrete chemical analysis of the soil in rocks to further identify and confirm the presence of other minerals. The only samples of Martian rocks that are on Earth are in the form of meteorites. The elemental and atmospheric composition along with planetary conditions is essential in knowing what minerals can be formed from these base parts.
This list gives an overview of the classification of non-silicate minerals and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, not IMA approved minerals, not named minerals are mostly excluded. Mostly major groups only, or groupings used by New Dana Classification and Mindat.
This list gives an overview of the classification of minerals (silicates) and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, non-IMA approved minerals and non-named minerals are mostly excluded.

Coupled substitution is the geological process by which two elements simultaneous substitute into a crystal in order to maintain overall electrical neutrality and keep the charge constant. In forming a solid solution series, ionic size is more important than ionic charge, as this can be compensated for elsewhere in the structure.