
Bioremediation broadly refers to any process wherein a biological system, living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer considerable advantages as it aims to be sustainable, eco-friendly, cheap, and scalable.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system also known as micro fuel cell that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs produce electricity by using the electrons derived from biochemical reactions catalyzed by bacteria.Comprehensive Biotechnology MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.
In biology, syntrophy, syntrophism, or cross-feeding is the cooperative interaction between at least two microbial species to degrade a single substrate. This type of biological interaction typically involves the transfer of one or more metabolic intermediates between two or more metabolically diverse microbial species living in close proximity to each other. Thus, syntrophy can be considered an obligatory interdependency and a mutualistic metabolism between different microbial species, wherein the growth of one partner depends on the nutrients, growth factors, or substrates provided by the other(s).

Shewanella is the sole genus included in the marine bacteria family Shewanellaceae. Some species within it were formerly classed as Alteromonas. Shewanella consists of facultatively anaerobic Gram-negative rods, most of which are found in extreme aquatic habitats where the temperature is very low and the pressure is very high. Shewanella bacteria are a normal component of the surface flora of fish and are implicated in fish spoilage. Shewanella chilikensis, a species of the genus Shewanella commonly found in the marine sponges of Saint Martin's Island of the Bay of Bengal, Bangladesh.
Microbial biodegradation is the use of bioremediation and biotransformation methods to harness the naturally occurring ability of microbial xenobiotic metabolism to degrade, transform or accumulate environmental pollutants, including hydrocarbons, polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), heterocyclic compounds, pharmaceutical substances, radionuclides and metals.

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
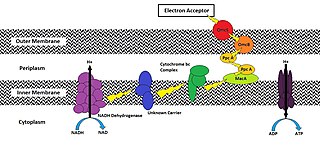
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
Geobacter metallireducens is a gram-negative metal-reducing proteobacterium. It is a strict anaerobe that oxidizes several short-chain fatty acids, alcohols, and monoaromatic compounds with Fe(III) as the sole electron acceptor. It can also use uranium for its growth and convert U(VI) to U(IV).
Geobacter sulfurreducens is a gram-negative metal and sulphur-reducing proteobacterium. It is rod-shaped, aerotolerant anaerobe, non-fermentative, has flagellum and type four pili, and is closely related to Geobacter metallireducens. Geobacter sulfurreducens is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, G. sulfurreducens is one out of twenty different species. The Geobacter genus was discovered by Derek R. Lovley in 1987. G. sulfurreducens was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
OmcS nanowires are conductive filaments found in some species of bacteria, including Geobacter sulfurreducens, where they catalyze the transfer of electrons. They are multiheme c-Type cytochromes localized outside of the cell of some exoelectrogenic bacterial species, serving as mediator of extracellular electron transfer from cells to Fe(III) oxides and other extracellular electron acceptors.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].

Forest rings are large, circular patterns of low tree density in the boreal forests of northern Canada. These rings can range from 50 metres (160 ft) to nearly 2 kilometres (1.2 mi) in diameter, with rims about 20 metres (66 ft) in thickness. The origin of forest rings is not known, despite several mechanisms for their creation having been proposed. Such hypotheses include radially growing fungus, buried kimberlite pipes, trapped gas pockets, and meteorite impact craters.
Geobacter anodireducens is a Gram-negative, aerotolerant, exoelectrogenic, anaerobic, non-spore-forming and non-motile bacterium from the genus of Geobacter Like others in its genus, it is commonly found in soil and uses iron as its electron acceptor. Due to its ability to generate current, it is an organism of note for Microbial fuel cell research. G. anodireducens was first isolated in 2014, and characterized in 2019, both by Dan Sun.
Geobacter daltonii is a Gram-negative, Fe(III)- and Uranium(IV)-reducing and non-spore-forming bacterium from the genus of Geobacter. It was isolated from sediments from the Oak Ridge Field Research Center in Oak Ridge, Tennessee in the United States. The specific epithet "daltonii" was refers to Dava Dalton, who performed the initial isolation of the strain, but died shortly thereafter.
Geobacter uraniireducens is a gram-negative, rod-shaped, anaerobic, chemolithotrophic, mesophilic, and motile bacterium from the genus of Geobacter. G. uraniireducens has been found to reduce iron and uranium in sediment and soil. It is being studied for use in bioremediation projects due to its ability to reduce uranium and arsenic.
Gemma Reguera is a Spanish-American microbiologist and professor at Michigan State University. She is the editor-in-chief of the journal Applied and Environmental Microbiology and was elected fellow of the American Academy of Microbiology in 2019. She is the recipient of the 2022 Alice C. Evans Award for Advancement of Women from the American Society for Microbiology. Her lab's research is focused on electrical properties of metal-reducing microorganisms.
Microbial electrochemical technologies (METs) use microorganisms as electrochemical catalyst, merging the microbial metabolism with electrochemical processes for the production of bioelectricity, biofuels, H2 and other valuable chemicals. Microbial fuel cells (MFC) and microbial electrolysis cells (MEC) are prominent examples of METs. While MFC is used to generate electricity from organic matter typically associated with wastewater treatment, MEC use electricity to drive chemical reactions such as the production of H2 or methane. Recently, microbial electrosynthesis cells (MES) have also emerged as a promising MET, where valuable chemicals can be produced in the cathode compartment. Other MET applications include microbial remediation cell, microbial desalination cell, microbial solar cell, microbial chemical cell, etc.,.






