Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Geobacter is a genus of bacteria. Geobacter species are anaerobic respiration bacterial species which have capabilities that make them useful in bioremediation. Geobacter was found to be the first organism with the ability to oxidize organic compounds and metals, including iron, radioactive metals, and petroleum compounds into environmentally benign carbon dioxide while using iron oxide or other available metals as electron acceptors. Geobacter species are also found to be able to respire upon a graphite electrode. They have been found in anaerobic conditions in soils and aquatic sediment.

Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate (SO2−
4) as terminal electron acceptor, reducing it to hydrogen sulfide (H2S). Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.
Ferroglobus is a genus of the Archaeoglobaceae.

Iron-oxidizing bacteria are chemotrophic bacteria that derive energy by oxidizing dissolved iron. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L. However, at least 0.3 ppm of dissolved oxygen is needed to carry out the oxidation.
Geoglobus is a hyperthermophilic member of the Archaeoglobaceae within the Euryarchaeota. It consists of two species, the first, G. ahangari, isolated from the Guaymas Basin hydrothermal system located deep within the Gulf of California. As a hyperthermophile, it grows best at a temperature of 88 °C and cannot grow at temperatures below 65 °C or above 90 °C. It possess an S-layer cell wall and a single flagellum. G. ahangari is an anaerobe, using poorly soluble ferric iron (Fe3+) as a terminal electron acceptor. It can grow either autotrophically using hydrogen gas (H2) or heterotrophically using a large number of organic compounds, including several types of fatty acids, as energy sources. G. ahangari was the first archaeon isolated capable of using hydrogen gas coupled to iron reduction as an energy source and the first anaerobe isolated capable of using long-chain fatty acids as an energy source.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
In biology, syntrophy, syntrophism, or cross-feeding is the cooperative interaction between at least two microbial species to degrade a single substrate. This type of biological interaction typically involves the transfer of one or more metabolic intermediates between two or more metabolically diverse microbial species living in close proximity to each other. Thus, syntrophy can be considered an obligatory interdependency and a mutualistic metabolism between different microbial species, wherein the growth of one partner depends on the nutrients, growth factors, or substrates provided by the other(s).
Shewanella putrefaciens is a Gram-negative pleomorphic bacterium. It has been isolated from marine environments, as well as from anaerobic sandstone in the Morrison Formation in New Mexico. S. putrefaciens is also a facultative anaerobe with the ability to reduce iron and manganese metabolically; that is, it can use iron and manganese as the terminal electron acceptor in the electron transport chain. It is also one of the organisms associated with the odor of rotting fish, as it is a marine organism which produces trimethylamine.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
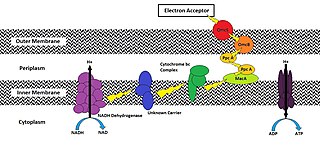
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.

Geothrix fermentans is a rod-shaped, anaerobic bacterium. It is about 0.1 µm in diameter and ranges from 2-3 µm in length. Cell arrangement occurs singly and in chains. Geothrix fermentans can normally be found in aquatic sediments such as in aquifers. As an anaerobic chemoorganotroph, this organism is best known for its ability to use electron acceptors Fe(III), as well as other high potential metals. It also uses a wide range of substrates as electron donors. Research on metal reduction by G. fermentans has contributed to understanding more about the geochemical cycling of metals in the environment.
Geobacter sulfurreducens is a gram-negative metal- and sulphur-reducing proteobacterium. It is rod-shaped, aerotolerant anaerobe, non-fermentative, has flagellum and type four pili, and is closely related to Geobacter metallireducens. Geobacter sulfurreducens is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, G. sulfurreducens is one out of twenty different species. The Geobacter genus was discovered by Derek R. Lovley in 1987. G. sulfurreducens was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
Geobacter psychrophilus is a Fe(III)-reducing bacterium. It is Gram-negative, slightly curved, rod-shaped and motile via means of monotrichous flagella. Its type strain is P35T.
OmcS nanowires are conductive filaments found in some species of bacteria, including Geobacter sulfurreducens, where they catalyze the transfer of electrons. They are multiheme c-Type cytochromes localized outside of the cell of some exoelectrogenic bacterial species, serving as mediator of extracellular electron transfer from cells to Fe(III) oxides and other extracellular electron acceptors.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].
Geobacter daltonii is a Gram-negative, Fe(III)- and Uranium(IV)-reducing and non-spore-forming bacterium from the genus of Geobacter. It was isolated from sediments from the Oak Ridge Field Research Center in Oak Ridge, Tennessee in the United States. The specific epithet "daltonii" was refers to Dava Dalton, who performed the initial isolation of the strain, but died shortly thereafter.
Geobacter uraniireducens is a gram-negative, rod-shaped, anaerobic, chemolithotrophic, mesophilic, and motile bacterium from the genus of Geobacter. G. uraniireducens has been found to reduce iron and uranium in sediment and soil. It is being studied for use in bioremediation projects due to its ability to reduce uranium and arsenic.
N-Methyliminodiacetic acid is an organic compound with the formula CH3N(CH2CO2H)2. It is a white solid, which as its conjugate base CH3N(CH2CO−2)2 is used as a chelating agent for iron. It is a component of organoboron reagents as well.






