
Limonite is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·nH2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the three principal iron ores, the others being hematite and magnetite, and has been mined for the production of iron since at least 2500 BP.

Goethite is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α-polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient times for its use as a pigment. Evidence has been found of its use in paint pigment samples taken from the caves of Lascaux in France. It was first described in 1806 based on samples found in the Hollertszug Mine in Herdorf, Germany. The mineral was named after the German polymath and poet Johann Wolfgang von Goethe (1749–1832).

Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the form of magnetite (Fe
3O
4, 72.4% Fe), hematite (Fe
2O
3, 69.9% Fe), goethite (FeO(OH), 62.9% Fe), limonite (FeO(OH)·n(H2O), 55% Fe) or siderite (FeCO3, 48.2% Fe).

Sphalerite is a sulfide mineral with the chemical formula (Zn,Fe)S. It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite, calcite, dolomite, quartz, rhodochrosite, and fluorite.

Ironstone is a sedimentary rock, either deposited directly as a ferruginous sediment or created by chemical replacement, that contains a substantial proportion of an iron ore compound from which iron (Fe) can be smelted commercially. Not to be confused with native or telluric iron, which is very rare and found in metallic form, the term ironstone is customarily restricted to hard, coarsely banded, non-banded, and non-cherty sedimentary rocks of post-Precambrian age. The Precambrian deposits, which have a different origin, are generally known as banded iron formations. The iron minerals comprising ironstones can consist either of oxides, i.e. limonite, hematite, and magnetite; carbonates, i.e. siderite; silicates, i.e. chamosite; or some combination of these minerals.

Rhodochrosite is a manganese carbonate mineral with chemical composition MnCO3. In its pure form (rare), it is typically a rose-red colour, but it can also be shades of pink to pale brown. It streaks white, and its Mohs hardness varies between 3.5 and 4.5. Its specific gravity is between 3.45 and 3.6. It crystallizes in the trigonal system, and cleaves with rhombohedral carbonate cleavage in three directions. The crystal structure of rhodochrosite is a rhombohedral system, which is a subset of the trigonal system. The carbonate ions (CO3) are arranged in a triangular planar configuration, and the manganese ions (Mn) are surrounded by six oxygen ions in an octahedral arrangement. The MnO6 octahedra and CO3 triangles are linked together to form a three-dimensional structure. Crystal twinning often is present. It is often confused with the manganese silicate, rhodonite, but is distinctly softer. Rhodochrosite is formed by the oxidation of manganese ore, and is found in South Africa, China, and the Americas. It is officially listed as one of the National symbols of Argentina.

Chiatura is a city in the Imereti region of Western Georgia. In 1989, it had a population of about 30,000. The city is known for its system of cable cars connecting the city's center to the mining settlements on the surrounding hills. The city is located inland, in a mountain valley on the banks of the Qvirila River.

Taconite is a variety of banded iron formation, an iron-bearing sedimentary rock, in which the iron minerals are interlayered with quartz, chert, or carbonate. The name "taconyte" was coined by Horace Vaughn Winchell (1865–1923) – son of Newton Horace Winchell, the Minnesota State Geologist – during their pioneering investigations of the Precambrian Biwabik Iron Formation of northeastern Minnesota. He believed the sedimentary rock sequence hosting the iron-formation was correlative with the Taconic orogeny of New England, and referred to the unfamiliar and as-yet-unnamed iron-bearing rock as the 'taconic rock' or taconyte.

Bog iron is a form of impure iron deposit that develops in bogs or swamps by the chemical or biochemical oxidation of iron carried in solution. In general, bog ores consist primarily of iron oxyhydroxides, commonly goethite.

Pyrolusite is a mineral consisting essentially of manganese dioxide (MnO2) and is important as an ore of manganese. It is a black, amorphous appearing mineral, often with a granular, fibrous, or columnar structure, sometimes forming reniform crusts. It has a metallic luster, a black or bluish-black streak, and readily soils the fingers. The specific gravity is about 4.8. Its name is from the Greek for fire and to wash, in reference to its use as a way to remove tints from glass.

Adamite is a zinc arsenate hydroxide mineral, Zn2AsO4OH. It is a mineral that typically occurs in the oxidized or weathered zone above zinc ore occurrences. Pure adamite is colorless, but usually it possess yellow color due to Fe compounds admixture. Tints of green also occur and are connected with copper substitutions in the mineral structure. Olivenite is a copper arsenate that is isostructural with adamite and there is considerable substitution between zinc and copper resulting in an intermediate called cuproadamite. Zincolivenite is a recently discovered mineral being an intermediate mineral with formula CuZn(AsO4)(OH). Manganese, cobalt, and nickel also substitute in the structure. An analogous zinc phosphate, tarbuttite, is known.
Wad is an old mining term for any black manganese oxide or hydroxide mineral-rich rock in the oxidized zone of various ore deposits. Typically closely associated with various iron oxides. Specific mineral varieties include pyrolusite, lithiophorite, nsutite, takanelite and vernadite. Wad can be considered to be the manganese equivalent to the iron mineraloid limonite.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

Ironsand, also known as iron-sand or iron sand, is a type of sand with heavy concentrations of iron. It is typically dark grey or blackish in colour.
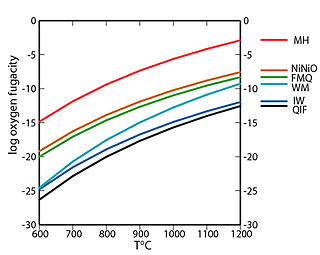
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

The Broken Hill Ore Deposit is located underneath Broken Hill in western New South Wales, Australia, and is the namesake for the town. It is arguably the world's richest and largest zinc-lead ore deposit.

The following outline is provided as an overview of and topical guide to mining:

Laterite is both a soil and a rock type rich in iron and aluminium and is commonly considered to have formed in hot and wet tropical areas. Nearly all laterites are of rusty-red coloration, because of high iron oxide content. They develop by intensive and prolonged weathering of the underlying parent rock, usually when there are conditions of high temperatures and heavy rainfall with alternate wet and dry periods. Tropical weathering (laterization) is a prolonged process of chemical weathering which produces a wide variety in the thickness, grade, chemistry and ore mineralogy of the resulting soils. The majority of the land area containing laterites is between the tropics of Cancer and Capricorn.

















