Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

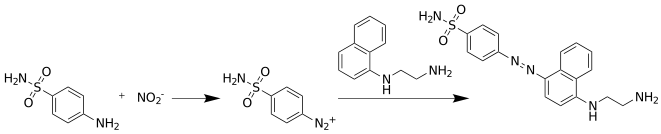
Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase and in the form of nitrite salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound and another aromatic compound that produces an azo compound. In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated carbon act as a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic.
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.
A nitrite test is a chemical test used to determine the presence of nitrite ion in solution.

Iodobenzene is an organoiodine compound consisting of a benzene ring substituted with one iodine atom. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish.
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance(s) present in a sample.
Frank Skuse is a British former forensic scientist for the North West Forensic Laboratories based in Chorley, Lancashire. His flawed conclusions, eventually discredited, contributed to the convictions of Judith Ward and the Birmingham Six.

Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.

Phenyl isothiocyanate (PITC) is a reagent used in reversed phase HPLC. PITC is less sensitive than o-phthaldehyde (OPA) and cannot be fully automated. PITC can be used for analysing secondary amines, unlike OPA. It is also known as Edman's reagent and is used in Edman degradation.

o-Anisidine (2-anisidine) is an organic compound with the formula CH3OC6H4NH2. A colorless liquid, commercial samples can appear yellow owing to air oxidation. It is one of three isomers of the methoxy-containing aniline derivative.

A urine test strip or dipstick is a basic diagnostic tool used to determine pathological changes in a patient's urine in standard urinalysis.

N-(1-Naphthyl)ethylenediamine is an organic compound. It is commercially available as part of Griess reagents, which find application in quantitative inorganic analysis of nitrates, nitrite and sulfonamide in blood, using the Griess test.
2,4-Dinitroaniline is a chemical compound with a formula of C6H5N3O4. It is used as an explosive and as a reagent to detect and characterize aldehydes and ketones.