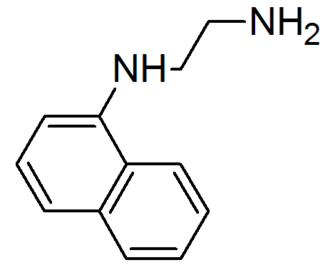Nitrate is an anion with the molecular formula NO−
3; or a salt with that anion. The name is also used for organic compounds that contain the nitrate ester functional group –ONO
2. Nitrates are common components of fertilizers and explosives. Almost all nitrate salts are soluble in water. A common example of an inorganic nitrate salt is potassium nitrate (saltpeter).
A urinary tract infection (UTI) is an infection that affects part of the urinary tract. When it affects the lower urinary tract it is known as a bladder infection (cystitis) and when it affects the upper urinary tract it is known as a kidney infection (pyelonephritis). Symptoms from a lower urinary tract infection include pain with urination, frequent urination, and feeling the need to urinate despite having an empty bladder. Symptoms of a kidney infection include fever and flank pain usually in addition to the symptoms of a lower UTI. Rarely the urine may appear bloody. In the very old and the very young, symptoms may be vague or non-specific.

The nitrite ion, which has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. Nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite can also refer to organic compounds with the -ONO group, which are esters of nitrous acid.

Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.

Serratia marcescens is a species of rod-shaped, Gram-negative bacteria, that is also a facultative anaerobic organism, classified as an opportunistic pathogen in the family Yersiniaceae. It was discovered in 1819 by Bartolomeo Bizio in Padua, Italy. S. marcescens is commonly involved in hospital-acquired infections (HAIs), particularly catheter-associated bacteremia, urinary tract infections, and wound infections, and is responsible for 1.4% of HAI cases in the United States. It is commonly found in the respiratory and urinary tracts of hospitalized adults and in the gastrointestinal systems of children. Due to its abundant presence in the environment, and its preference for damp conditions, S. marcescens is commonly found growing in bathrooms, where it manifests as a pink, pink-orange, or orange discoloration and slimy film feeding off phosphorus-containing materials or fatty substances such as soap and shampoo residue.

Proteus vulgaris is a rod-shaped, nitrate-reducing, indole-positive and catalase-positive, hydrogen sulfide-producing, Gram-negative bacterium that inhabits the intestinal tracts of humans and animals. It can be found in soil, water, and fecal matter. It is grouped with the Morganellaceae and is an opportunistic pathogen of humans. It is known to cause wound infections and other species of its genera are known to cause urinary tract infections.

Proteus mirabilis is a Gram-negative, facultatively anaerobic, rod-shaped bacterium. It shows swarming motility and urease activity. P. mirabilis causes 90% of all Proteus infections in humans. It is widely distributed in soil and water. Proteus mirabilis can migrate across the surface of solid media or devices using a type of cooperative group motility called swarming. Proteus mirabilis is most frequently associated with infections of the urinary tract, especially in complicated or catheter-associated urinary tract infections.

Pyelonephritis is inflammation of the kidney, typically due to a bacterial infection. Symptoms most often include fever and flank tenderness. Other symptoms may include nausea, burning with urination, and frequent urination. Complications may include pus around the kidney, sepsis, or kidney failure.
Staphylococcus saprophyticus is a Gram-positive coccus belonging to the genus Staphylococcus. S. saprophyticus is a common cause of community-acquired urinary tract infections.
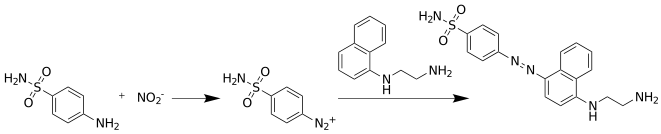
The Griess test is an analytical chemistry test which detects the presence of nitrite ion in solution. One of its most important uses is the determination of nitrite in drinking water. The Griess diazotization reaction, on which the Griess reagent relies, was first described in 1858 by Peter Griess. The test has also been widely used for the detection of nitrates, which are common component of explosives, as they can be converted to nitrites and detected with the Griess test.

Pyuria is the condition of urine containing white blood cells or pus. Defined as the presence of 6-10 or more neutrophils per high power field of unspun, voided mid-stream urine, it can be a sign of a bacterial urinary tract infection. Pyuria may be present in the people with sepsis, or in older people with pneumonia. Others additionally require discoloration, clouding or change in the smell of urine for a pyuria to be present. Without these additional features, there is said to be leukocyturia.

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).
Leukocyte esterase (LE) is an esterase produced by leukocytes. A leukocyte esterase test is a urine test for the presence of white blood cells and other abnormalities associated with infection.
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.

Bacteriuria is the presence of bacteria in urine. Bacteriuria accompanied by symptoms is a urinary tract infection while that without is known as asymptomatic bacteriuria. Diagnosis is by urinalysis or urine culture. Escherichia coli is the most common bacterium found. People without symptoms should generally not be tested for the condition. Differential diagnosis include contamination.
Nitric oxide is a molecule and chemical compound with chemical formula of NO. In mammals including humans, nitric oxide is a signaling molecule involved in many physiological and pathological processes. It is a powerful vasodilator with a half-life of a few seconds in the blood. Standard pharmaceuticals such as nitroglycerine and amyl nitrite are precursors to nitric oxide. Low levels of nitric oxide production are typically due to ischemic damage in the liver.

A urine test strip or dipstick is a basic diagnostic tool used to determine pathological changes in a patient's urine in standard urinalysis.

Citrobacter freundii is a species of facultative anaerobic gram-negative bacteria of the family Enterobacteriaceae. The bacteria have a long rod shape with a typical length of 1–5 μm. Most C. freundii cells generally have several flagella used for locomotion, but some do not and are non-motile. C. freundii is a soil organism, but can also be found in water, sewage, food and in the intestinal tracts of animals and humans. The genus Citrobacter was discovered in 1932 by Werkman and Gillen. Cultures of C. freundii were isolated and identified in the same year from soil extracts.

Saliva testing or Salivaomics is a diagnostic technique that involves laboratory analysis of saliva to identify markers of endocrine, immunologic, inflammatory, infectious, and other types of conditions. Saliva is a useful biological fluid for assaying steroid hormones such as cortisol, genetic material like RNA, proteins such as enzymes and antibodies, and a variety of other substances, including natural metabolites, including saliva nitrite, a biomarker for nitric oxide status. Saliva testing is used to screen for or diagnose numerous conditions and disease states, including Cushing's disease, anovulation, HIV, cancer, parasites, hypogonadism, and allergies. Salivary testing has even been used by the U.S. government to assess circadian rhythm shifts in astronauts before flight and to evaluate hormonal profiles of soldiers undergoing military survival training.
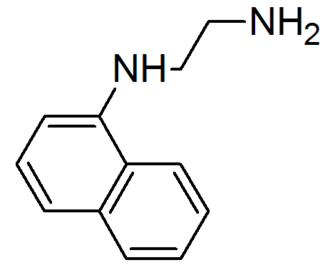
N-(1-Naphthyl)ethylenediamine is an organic compound. It is commercially available as part of Griess reagents, which find application in quantitative inorganic analysis of nitrates, nitrite and sulfonamide in blood, using the Griess test.