
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, diabetes mellitus type 1, Henoch–Schönlein purpura (HSP), systemic lupus erythematosus (SLE), Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis (RA), ankylosing spondylitis, polymyositis (PM), dermatomyositis (DM), and multiple sclerosis (MS). Autoimmune diseases are very often treated with steroids.

The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are called MHC molecules.

The human leukocyte antigen (HLA) system or complex is a complex of genes on chromosome 6 in humans which encode cell-surface proteins responsible for regulation of the immune system. The HLA system is also known as the human version of the major histocompatibility complex (MHC) found in many animals.

Ankylosing spondylitis (AS) is a type of arthritis characterized by long-term inflammation of the joints of the spine, typically where the spine joins the pelvis. With AS, eye, bowel problems and back pain may occur. Joint mobility in the affected areas sometimes worsens over time. Ankylosing spondylitis is believed to involve a combination of genetic and environmental factors. More than 85% of people affected in the UK have a specific human leukocyte antigen known as the HLA-B27 antigen. The underlying mechanism is believed to be autoimmune or autoinflammatory. Diagnosis is based on symptoms with support from medical imaging and blood tests. AS is a type of seronegative spondyloarthropathy, meaning that tests show no presence of rheumatoid factor (RF) antibodies.
Spondyloarthropathy or spondyloarthrosis refers to any joint disease of the vertebral column. As such, it is a class or category of diseases rather than a single, specific entity. It differs from spondylopathy, which is a disease of the vertebra itself, but many conditions involve both spondylopathy and spondyloarthropathy.
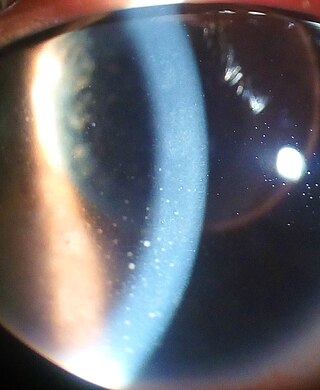
Uveitis is inflammation of the uvea, the pigmented layer of the eye between the inner retina and the outer fibrous layer composed of the sclera and cornea. The uvea consists of the middle layer of pigmented vascular structures of the eye and includes the iris, ciliary body, and choroid. Uveitis is described anatomically, by the part of the eye affected, as anterior, intermediate or posterior, or panuveitic if all parts are involved. Anterior uveitis (iridocyclitis) is the most common, with the incidence of uveitis overall affecting approximately 1:4500, most commonly those between the ages of 20-60. Symptoms include eye pain, eye redness, floaters and blurred vision, and ophthalmic examination may show dilated ciliary blood vessels and the presence of cells in the anterior chamber. Uveitis may arise spontaneously, have a genetic component, or be associated with an autoimmune disease or infection. While the eye is a relatively protected environment, its immune mechanisms may be overcome resulting in inflammation and tissue destruction associated with T-cell activation.
Reactive arthritis, also known as Reiter's syndrome, is a form of inflammatory arthritis that develops in response to an infection in another part of the body (cross-reactivity). Coming into contact with bacteria and developing an infection can trigger the disease. By the time the patient presents with symptoms, often the "trigger" infection has been cured or is in remission in chronic cases, thus making determination of the initial cause difficult.
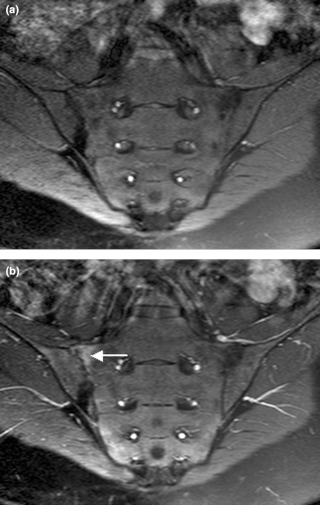
Sacroiliitis is inflammation within the sacroiliac joint. It is a feature of spondyloarthropathies, such as axial spondyloarthritis, psoriatic arthritis, reactive arthritis or arthritis related to inflammatory bowel diseases, including ulcerative colitis or Crohn's disease. It is also the most common presentation of arthritis from brucellosis.

β2 microglobulin (B2M) is a component of MHC class I molecules. MHC class I molecules have α1, α2, and α3 proteins which are present on all nucleated cells. In humans, the β2 microglobulin protein is encoded by the B2M gene.
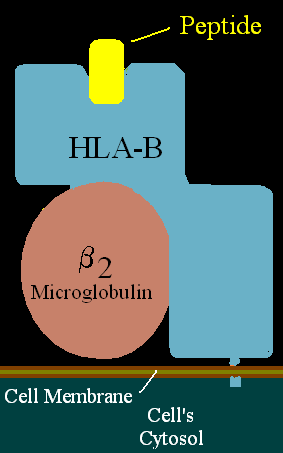
HLA-B is a human gene that provides instructions for making a protein that plays a critical role in the immune system. HLA-B is part of a family of genes called the human leukocyte antigen (HLA) complex. The HLA complex helps the immune system distinguish the body's own proteins from proteins made by foreign invaders such as viruses and bacteria.
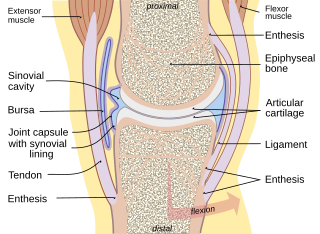
Enthesitis is inflammation of the entheses, the sites where tendons or ligaments insert into the bone. It is an enthesopathy, a pathologic condition of the entheses. Early clinical manifestations are an aching sensation akin to "working out too much", and it gets better with activity. It is worse in the morning. The muscle insertion hurts very focally as it joins into the bone, but there is little to no pain at all with passive motion. There are some cases of isolated, primary enthesitis which are very poorly studied and understood. It is known to be associated with other autoimmune diseases, like spondyloarthropathies and psoriasis. A common autoimmune enthesitis is at the heel, where the Achilles tendon attaches to the calcaneus.
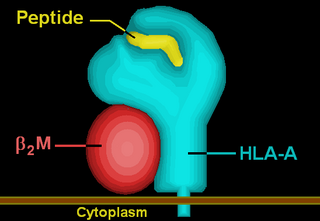
HLA-A is a group of human leukocyte antigens (HLA) that are encoded by the HLA-A locus, which is located at human chromosome 6p21.3. HLA is a major histocompatibility complex (MHC) antigen specific to humans. HLA-A is one of three major types of human MHC class I transmembrane proteins. The others are HLA-B and HLA-C. The protein is a heterodimer, and is composed of a heavy α chain and smaller β chain. The α chain is encoded by a variant HLA-A gene, and the β chain (β2-microglobulin) is an invariant β2 microglobulin molecule. The β2 microglobulin protein is encoded by the B2M gene, which is located at chromosome 15q21.1 in humans.

HLA-A*02 (A*02) is a human leukocyte antigen serotype within the HLA-A serotype group. The serotype is determined by the antibody recognition of the α2 domain of the HLA-A α-chain. For A*02, the α chain is encoded by the HLA-A*02 gene and the β chain is encoded by the B2M locus. In 2010 the World Health Organization Naming Committee for Factors of the HLA System revised the nomenclature for HLAs. Before this revision, HLA-A*02 was also referred to as HLA-A2, HLA-A02, and HLA-A*2.
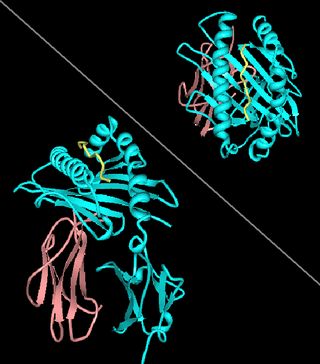
HLA-A24 (A24) is a human leukocyte antigen serotype within HLA-A serotype group. The serotype is determined by the antibody recognition of α24 subset of HLA-A α-chains. For A24, the alpha, "A", chain are encoded by the HLA-A*24 allele group and the β-chain are encoded by B2M locus. This group currently is dominated by A*2402. A24 and A*24 are almost synonymous in meaning. A24 is a split antigen of the broad antigen HLA-A9 and it is a sister serotype of HLA-A23.

HLA class I histocompatibility antigen, alpha chain F is a protein that in humans is encoded by the HLA-F gene. It is an empty intracellular molecule that encodes a non-classical heavy chain anchored to the membrane and forming a heterodimer with a β-2 microglobulin light chain. It belongs to the HLA class I heavy chain paralogues that separate from most of the HLA heavy chains. HLA-F is localized in the endoplasmic reticulum and Golgi apparatus, and is also unique in the sense that it exhibits few polymorphisms in the human population relative to the other HLA genes; however, there have been found different isoforms from numerous transcript variants found for the HLA-F gene. Its pathways include IFN-gamma signaling and CDK-mediated phosphorylation and removal of the Saccharomycescerevisiae Cdc6 protein, which is crucial for functional DNA replication.
Major histocompatibility complex class I-related gene protein (MR1) is a non-classical MHC class I protein, that binds vitamine metabolites produced in certain types of bacteria. MR1 interacts with mucosal associated invariant T cells (MAIT).

HLA-B39 (B39) is an HLA-B serotype. The serotype identifies the more common HLA-B*39 gene products.

Enteropathic arthropathy commonly referred to as enteropathic arthritis, is a type of arthritis linked to Crohn's disease, ulcerative colitis, and chronic inflammatory bowel diseases.

Axial spondyloarthritis is a chronic, immune-mediated disease predominantly affecting the axial skeleton. The term itself is an umbrella term characterizing a diverse disease family united by shared clinical and genetic features, such as the involvement of the axial skeleton. The 2009 introduced term axial spondyloarthritis is a preferred term nowadays and substitutes the old term ankylosing spondylitis.

Interleukin 40 (IL-40), also known with other name C17orf99, is a protein belonging to a group of cytokines called interleukins. It is encoded by a gene that does not belong to any cytokine superfamily. This cytokine is produced primarily by human expression tissues such as bone marrow and fetal liver, and its expression can be also induced in peripheral B cells after activation. IL-40 is involved in immunoglobulin A (IgA) production, and plays an important role in humoral immune responses and B cell homeostasis and development.


















