
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy, making it one of the last two stable elements to be discovered. Hafnium is named after Hafnia, the Latin name for Copenhagen, where it was discovered.
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of gamma radiation by atomic nuclei bound in a solid. Its main application is in Mössbauer spectroscopy.

Compton scattering is the quantum theory of high frequency photons scattering following an interaction with a charged particle, usually an electron. Specifically, when the photon hits electrons, it releases loosely bound electrons from the outer valence shells of atoms or molecules.
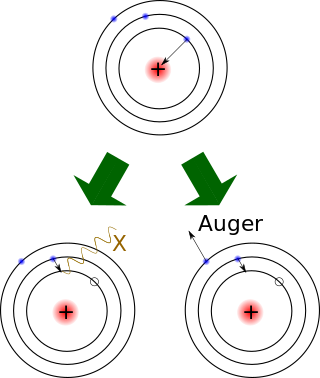
Electron capture is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.

A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state (higher energy) levels. "Metastable" describes nuclei whose excited states have half-lives 100 to 1000 times longer than the half-lives of the excited nuclear states that decay with a "prompt" half life (ordinarily on the order of 10−12 seconds). The term "metastable" is usually restricted to isomers with half-lives of 10−9 seconds or longer. Some references recommend 5 × 10−9 seconds to distinguish the metastable half life from the normal "prompt" gamma-emission half-life. Occasionally the half-lives are far longer than this and can last minutes, hours, or years. For example, the 180m
73Ta
nuclear isomer survives so long (at least 1015 years) that it has never been observed to decay spontaneously. The half-life of a nuclear isomer can even exceed that of the ground state of the same nuclide, as shown by 180m
73Ta
as well as 186m
75Re
, 192m2
77Ir
, 210m
83Bi
, 212m
84Po
, 242m
95Am
and multiple holmium isomers.
An axion is a hypothetical elementary particle originally postulated by the Peccei–Quinn theory in 1977 to resolve the strong CP problem in quantum chromodynamics (QCD). If axions exist and have low mass within a specific range, they are of interest as a possible component of cold dark matter.
In physics, induced gamma emission (IGE) refers to the process of fluorescent emission of gamma rays from excited nuclei, usually involving a specific nuclear isomer. It is analogous to conventional fluorescence, which is defined as the emission of a photon by an excited electron in an atom or molecule. In the case of IGE, nuclear isomers can store significant amounts of excitation energy for times long enough for them to serve as nuclear fluorescent materials. There are over 800 known nuclear isomers but almost all are too intrinsically radioactive to be considered for applications. As of 2006 there were two proposed nuclear isomers that appeared to be physically capable of IGE fluorescence in safe arrangements: tantalum-180m and hafnium-178m2.
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is relatively stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the generally accepted age of the universe. This isotope makes up nearly all natural thorium, so thorium was considered to be mononuclidic. However, in 2013, IUPAC reclassified thorium as binuclidic, due to large amounts of 230Th in deep seawater. Thorium has a characteristic terrestrial isotopic composition and thus a standard atomic weight can be given.
Natural hafnium (72Hf) consists of five observationally stable isotopes (176Hf, 177Hf, 178Hf, 179Hf, and 180Hf) and one very long-lived radioisotope, 174Hf, with a half-life of 7.0×1016 years. In addition, there are 34 known synthetic radioisotopes, the most stable of which is 182Hf with a half-life of 8.9×106 years. This extinct radionuclide is used in hafnium–tungsten dating to study the chronology of planetary differentiation.
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. Thirteen radioisotopes are known, ranging from 255Db to 270Db, along with one isomer (257mDb); two more isomers have been reported but are unconfirmed. The longest-lived known isotope is 268Db with a half-life of 16 hours.
Hassium (108Hs) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 265Hs in 1984. There are 13 known isotopes from 263Hs to 277Hs and 1–4 isomers. The most stable isotope of hassium cannot be determined based on existing data due to uncertainty that arises from the low number of measurements. The half-lives of 269Hs and 271Hs are about 12 seconds, whereas that of 270Hs is about 7.6 seconds. It is also possible that 277mHs is more stable than these, with its half-life likely being 130±100 seconds, but only one event of decay of this isotope has been registered as of 2016.
Darmstadtium (110Ds) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 269Ds in 1994. There are 11 known radioisotopes from 267Ds to 281Ds and 2 or 3 known isomers. The longest-lived isotope is 281Ds with a half-life of 14 seconds.
Copernicium (112Cn) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 277Cn in 1996. There are 6 known radioisotopes ; the longest-lived isotope is 285Cn with a half-life of 30 seconds.
Yrast is a technical term in nuclear physics that refers to a state of a nucleus with a minimum of energy for a given angular momentum. Yr is a Swedish adjective sharing the same root as the English whirl. Yrast is the superlative of yr and can be translated whirlingest, although it literally means "dizziest" or "most bewildered". The yrast levels are vital to understanding reactions, such as off-center heavy ion collisions, that result in high-spin states.

A nuclear clock or nuclear optical clock is a notional clock that would use the frequency of a nuclear transition as its reference frequency, in the same manner as an atomic clock uses the frequency of an electronic transition in an atom's shell. Such a clock is expected to be more accurate than the best current atomic clocks by a factor of about 10, with an achievable accuracy approaching the 10−19 level. The only nuclear state suitable for the development of a nuclear clock using existing technology is thorium-229m, a nuclear isomer of thorium-229 and the lowest-energy nuclear isomer known. With an energy of 8.35574(3) eV, this corresponds to a wavelength of 148.3821(5) nm in the vacuum ultraviolet region, making it accessible to laser excitation. A comprehensive review can be found in reference.
The isomeric shift is the shift on atomic spectral lines and gamma spectral lines, which occurs as a consequence of replacement of one nuclear isomer by another. It is usually called isomeric shift on atomic spectral lines and Mössbauer isomeric shift respectively. If the spectra also have hyperfine structure the shift refers to the center of gravity of the spectra. The isomeric shift provides important information about the nuclear structure and the physical, chemical or biological environment of atoms. More recently the effect has also been proposed as a tool in the search for the time variation of fundamental constants of nature.

Photofission is a process in which a nucleus, after absorbing a gamma ray, undergoes nuclear fission and splits into two or more fragments.

The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron.

The Breit–Wheeler process or Breit–Wheeler pair production is a proposed physical process in which a positron–electron pair is created from the collision of two photons. It is the simplest mechanism by which pure light can be potentially transformed into matter. The process can take the form γ γ′ → e+ e− where γ and γ′ are two light quanta.
George Curriden Baldwin was an American theoretical and experimental physicist. He was a professor of nuclear engineering at Rensselaer Polytechnic Institute and a scientist working at the General Electric Research Laboratory and at the Los Alamos National Laboratory. He wrote a book on Nonlinear Optics and authored or co-authored over 130 technical papers.






