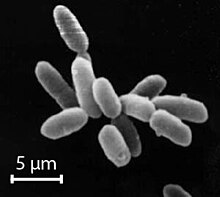
The halophiles, named after the Greek word for "salt-loving", are extremophiles that thrive in high salt concentrations. While most halophiles are classified into the domain Archaea, there are also bacterial halophiles and some eukaryotic species, such as the alga Dunaliella salina and fungus Wallemia ichthyophaga. Some well-known species give off a red color from carotenoid compounds, notably bacteriorhodopsin. Halophiles can be found in water bodies with salt concentration more than five times greater than that of the ocean, such as the Great Salt Lake in Utah, Owens Lake in California, the Urmia Lake in Iran, the Dead Sea, and in evaporation ponds. They are theorized to be a possible analogues for modeling extremophiles that might live in the salty subsurface water ocean of Jupiter's Europa and similar moons.

The phylum Bacteroidota is composed of three large classes of Gram-negative, nonsporeforming, anaerobic or aerobic, and rod-shaped bacteria that are widely distributed in the environment, including in soil, sediments, and sea water, as well as in the guts and on the skin of animals.
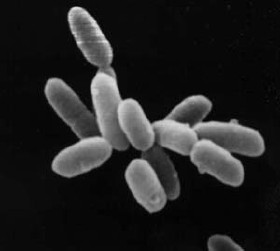
Halobacterium is a genus in the family Halobacteriaceae.
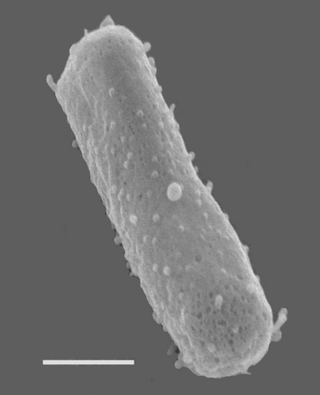
Halobacterium salinarum, formerly known as Halobacterium cutirubrum or Halobacterium halobium, is an extremely halophilic marine obligate aerobic archaeon. Despite its name, this is not a bacterium, but a member of the domain Archaea. It is found in salted fish, hides, hypersaline lakes, and salterns. As these salterns reach the minimum salinity limits for extreme halophiles, their waters become purple or reddish color due to the high densities of halophilic Archaea. H. salinarum has also been found in high-salt food such as salt pork, marine fish, and sausages. The ability of H. salinarum to survive at such high salt concentrations has led to its classification as an extremophile.
Halorubrum is a genus in the family Halorubraceae. Halorubrum species areusually halophilic and can be found in waters with high salt concentration such as the Dead Sea or Lake Zabuye.
Archaeocin is the name given to a new type of potentially useful antibiotic that is derived from the Archaea group of organisms. Eight archaeocins have been partially or fully characterized, but hundreds of archaeocins are believed to exist, especially within the haloarchaea. Production of these archaeal proteinaceous antimicrobials is a nearly universal feature of the rod-shaped haloarchaea.

Haloferax volcanii is a species of organism in the genus Haloferax in the Archaea.

Haloquadratum walsbyi is of the genus Haloquadratum, within the archaea domain known for its square halophilic nature. First discovered in a brine pool in the Sinai peninsula of Egypt, H. walsbyi is noted for its flat, square-shaped cells, and its unusual ability to survive in aqueous environments with high concentrations of sodium chloride and magnesium chloride. The species' genus name Haloquadratum translates from Greek and Latin as "salt square". This archaean is also commonly referred to as "Walsby's Square Bacterium" because of its identifying square shape which makes it unique. In accordance with its name, Haloquadratum walsbyi are most abundantly observed in salty environments.

Methanohalophilus mahii is an obligately anaerobic, methylotrophic, methanogenic cocci-shaped archaeon of the genus Methanohalophilus that can be found in high salinity aquatic environments. The name Methanohalophilus is said to be derived from methanum meaning "methane" in Latin; halo meaning "salt" in Greek; and mahii meaning "of Mah" in Latin, after R.A. Mah, who did substantial amounts of research on aerobic and methanogenic microbes. The proper word in ancient Greek for "salt" is however hals (ἅλς). The specific strain type was designated SLP and is currently the only identified strain of this species.
Halostagnicola larsenii is a non-motile, aerobic, gram-negative, rod shaped archaeon. It is a halophilic, neutrophilic, chemo-organotroph and was isolated from samples taken from a saline lake in China. The etymology of the name comes from hals, halos Greek for salt, stagnum Latin for a piece of standing water, -cola Latin for inhabitant or dweller, and Larsenii named after the Norwegian microbiologist, Helge Larsen, who was a pioneer in research regarding halophiles.
Salinibacter ruber is an extremely halophilic red bacterium, first found in Spain in 2002.
Haladaptatus paucihalophilus is a halophilic archaeal species, originally isolated from a spring in Oklahoma. It uses a new pathway to synthesize glycine, and contains unique physiological features for osmoadaptation.

Shiladitya DasSarma is a molecular biologist well-known for contributions to the biology of halophilic and extremophilic microorganisms. He is a Professor in the University of Maryland Baltimore. He earned a PhD degree in Biochemistry from the Massachusetts Institute of Technology and a BS degree in Chemistry from Indiana University Bloomington. Prior to taking a faculty position, he conducted research at the Massachusetts General Hospital, Harvard Medical School, and Pasteur Institute, Paris.
Haloterrigena turkmenica is an aerobic chemo organotrophic archeon originally found in Turkish salt lakes.
Halomonas meridiana is a bacterial species discovered in 1990 in the hypersaline lakes of Vestfold Hills, Antarctica.
Haloarcula marismortui is a halophilic archaeon isolated from the Dead Sea
Halorubrum lacusprofundi is a rod-shaped, halophilic Archaeon in the family of Halorubraceae. It was first isolated from Deep Lake in Antarctica in the 1980s.
Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian GPCR protein rhodopsin, but are not true homologs.

An archaeal virus is a virus that infects and replicates in archaea, a domain of unicellular, prokaryotic organisms. Archaeal viruses, like their hosts, are found worldwide, including in extreme environments inhospitable to most life such as acidic hot springs, highly saline bodies of water, and at the bottom of the ocean. They have been also found in the human body. The first known archaeal virus was described in 1974 and since then, a large diversity of archaeal viruses have been discovered, many possessing unique characteristics not found in other viruses. Little is known about their biological processes, such as how they replicate, but they are believed to have many independent origins, some of which likely predate the last archaeal common ancestor (LACA).