
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene.

Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 464-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (thrombin) and factor Xa.

Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases. They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and, in the treatment of venous thromboembolism, and the treatment of myocardial infarction.

Protein S is a vitamin K-dependent plasma glycoprotein synthesized in the liver. In the circulation, Protein S exists in two forms: a free form and a complex form bound to complement protein C4b-binding protein (C4BP). In humans, protein S is encoded by the PROS1 gene. Protein S plays a role in coagulation.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.

Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.

Hirudin is a naturally occurring peptide in the salivary glands of blood-sucking leeches that has a blood anticoagulant property. This is essential for the leeches' habit of feeding on blood, since it keeps a host's blood flowing after the worm's initial puncture of the skin.
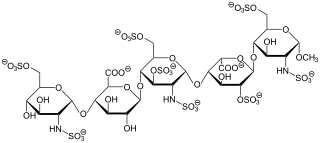
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.
The prothrombinase enzyme complex consists of factor Xa (a serine protease) and factor Va (a protein cofactor). The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (factor II), an inactive zymogen, to thrombin (factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
Danaparoid sodium (Orgaran) is an anticoagulant with an antithrombotic action due to inhibition of thrombin generation (TGI) by two mechanisms: indirect inactivation of Factor Xa via AT and direct inhibition of thrombin activation of Factor IX. It also possesses a minor anti-thrombin activity, mediated equally via AT and Heparin Co-factor II producing a ratio of anti-Xa:IIa activity >22. [Meuleman DG. Haemostasis 1992;22:58-65 and Ofosu FA Haemostasis 1992;22:66-72]

Bivalirudin, sold under the brand names Angiomax and Angiox, among others, is a specific and reversible direct thrombin inhibitor (DTI). Chemically, it is a synthetic congener of the naturally occurring drug hirudin, found in the saliva of the medicinal leech Hirudo medicinalis. It is manufactured by The Medicines Company.

Platelet factor 4 (PF4) is a small cytokine belonging to the CXC chemokine family that is also known as chemokine ligand 4 (CXCL4). This chemokine is released from alpha-granules of activated platelets during platelet aggregation, and promotes blood coagulation by moderating the effects of heparin-like molecules. Due to these roles, it is predicted to play a role in wound repair and inflammation. It is usually found in a complex with proteoglycan.

Protein C inhibitor is a serine protease inhibitor (serpin) that limits the activity of protein C.

β2-glycoprotein 1, also known as beta-2 glycoprotein 1 and Apolipoprotein H (Apo-H), is a 38 kDa multifunctional plasma protein that in humans is encoded by the APOH gene. One of its functions is to bind cardiolipin. When bound, the structure of cardiolipin and β2-GP1 both undergo large changes in structure. Within the structure of Apo-H is a stretch of positively charged amino acids, Lys-Asn-Lys-Glu-Lys-Lys, are involved in phospholipid binding.

Anti-thrombin antibodies are autoantibodies directed against thrombin that may constitute a fraction of lupus anticoagulant and are seen an increased levels in systemic lupus erythematosus.

Protease activated receptor 3 (PAR-3) also known as coagulation factor II receptor-like 2 (F2RL2) and thrombin receptor-like 2, is a protein that in humans is encoded by the F2RL2 gene.
Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene CPB2.























