In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

Acetonitrile, often abbreviated MeCN, is the chemical compound with the formula CH3CN and structure H3C−C≡N. This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The N≡C−C skeleton is linear with a short C≡N distance of 1.16 Å.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution.
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation.
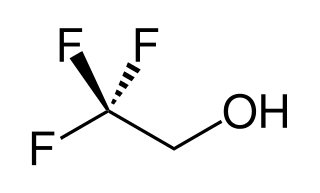
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol.
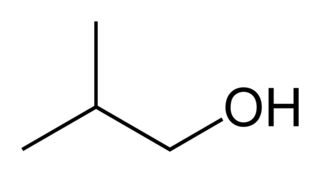
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is also used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.

1-Propanol is a primary alcohol with the formula CH3CH2CH2OH and sometimes represented as PrOH or n-PrOH. It is a colourless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.

Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour. The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol (F
3C)
2C(OH)
2, a geminal diol.
The Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1941 while studying the rearrangements of allyl vinyl ketones.
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. The enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes.
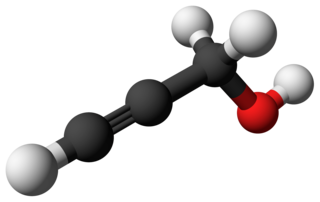
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. They all have the formula C3H8O.
Within the area of organocatalysis, (thio)urea organocatalysis describes the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. The effects arise through hydrogen-bonding interactions between the substrate and the (thio)urea. Unlike classical catalysts, these organocatalysts interact by non-covalent interactions, especially hydrogen bonding. The scope of these small-molecule H-bond donors termed (thio)urea organocatalysis covers both non-stereoselective and stereoselective applications.
Base-promoted epoxide isomerization is the conversion of alkyl epoxides to ring-opened products through the action of strong base. Isomerizations of this type are most often used to synthesize allylic alcohols, although other products are possible.
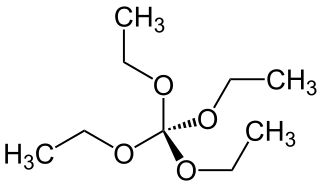
Tetraethoxymethane is a chemical compound which is formally formed by complete ethylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarbonic acid violates the Erlenmeyer rule and is unstable in free state).
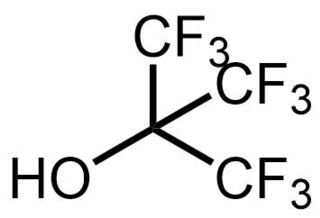
Nonafluoro-tert-butyl alcohol is a fluoroalcohol. It is the perfluorinated analog of tert-butyl alcohol. Notably, as a consequence of its electron withdrawing fluorine substituents, it is very acidic for an alcohol, with a pKa value of 5.4, similar to that of a carboxylic acid. As another consequence of being a perfluorinated compound, it is also one of the lowest boiling alcohols, with a boiling point lower than that of methanol.