A urinary tract infection (UTI) is an infection that affects a part of the urinary tract. Lower urinary tract infections may involve the bladder (cystitis) or urethra (urethritis) while upper urinary tract infections affect the kidney (pyelonephritis). Symptoms from a lower urinary tract infection include suprapubic pain, painful urination (dysuria), frequency and urgency of urination despite having an empty bladder. Symptoms of a kidney infection, on the other hand, are more systemic and include fever or flank pain usually in addition to the symptoms of a lower UTI. Rarely, the urine may appear bloody. Symptoms may be vague or non-specific at the extremities of age.

Methylthioninium chloride, commonly called methylene blue, is a salt used as a dye and as a medication. As a medication, it is mainly used to treat methemoglobinemia by chemically reducing the ferric iron in hemoglobin to ferrous iron. Specifically, it is used to treat methemoglobin levels that are greater than 30% or in which there are symptoms despite oxygen therapy. It has previously been used for treating cyanide poisoning and urinary tract infections, but this use is no longer recommended.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
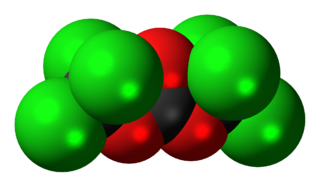
Triphosgene (bis(trichloromethyl) carbonate (BTC) is a chemical compound with the formula OC(OCCl3)2. It is used as a solid substitute for phosgene, which is a gas and diphosgene, which is a liquid. Triphosgene is stable up to 200 °C. Triphosgene is used in a variety of halogenation reactions.

Pyelonephritis is inflammation of the kidney, typically due to a bacterial infection. Symptoms most often include fever and flank tenderness. Other symptoms may include nausea, burning with urination, and frequent urination. Complications may include pus around the kidney, sepsis, or kidney failure.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.

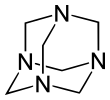
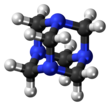
A hexamine fuel tablet is a form of solid fuel in tablet form. The tablets burn smokelessly, have a high energy density, do not liquefy while burning and leave no ashes. Invented in Murrhardt, Germany, in 1936, the main component is hexamine, which was discovered by Aleksandr Butlerov in 1859. Some fuel tablets use 1,3,5-trioxane as another ingredient.
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after James Cooper Duff.
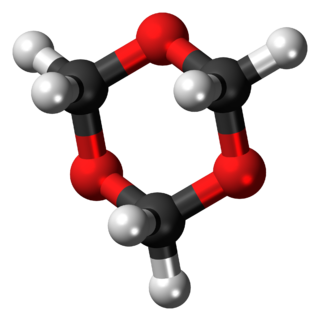
1,3,5-Trioxane, sometimes also called trioxane or trioxin, is a chemical compound with molecular formula C3H6O3. It is a white, highly water-soluble solid with a chloroform-like odor. It is a stable cyclic trimer of formaldehyde, and one of the three trioxane isomers; its molecular backbone consists of a six-membered ring with three carbon atoms alternating with three oxygen atoms.
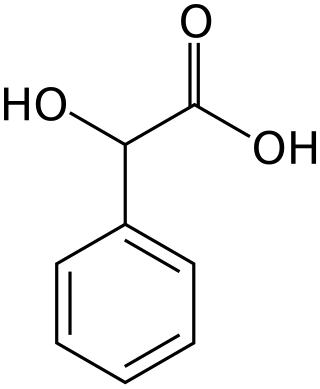
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.

Palladium on carbon, often referred to as Pd/C, is a form of palladium used as a catalyst. The metal is supported on activated carbon to maximize its surface area and activity.
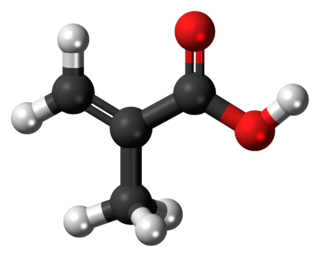
Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. Two abbreviation for pivalic acid are t-BuC(O)OH and PivOH. The pivalyl or pivaloyl group is abbreviated t-BuC(O).

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. It is also an important pheromone in certain species.
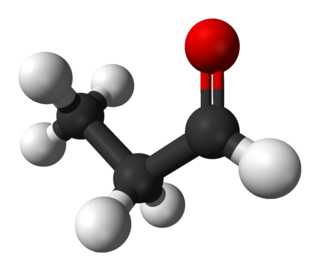
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.

1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.

Propionyl chloride (also propanoyl chloride) is the organic compound with the formula CH3CH2C(O)Cl. It is the acyl chloride derivative of propionic acid. It undergoes the characteristic reactions of acyl chlorides. It is a colorless, corrosive, volatile liquid.
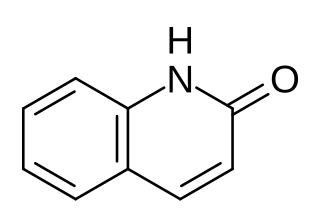
2-Quinolone is an organic compound related structurally to quinoline. It is the majority tautomer in equilibrium with 2-quinolinol. The compound can be classified as a cyclic amide, and as such is used as an isostere for peptides and other pharmaceutically inspired targets. The 4-methyl-2-quinolone can be prepared by dehydration of acetoacetanilide.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.
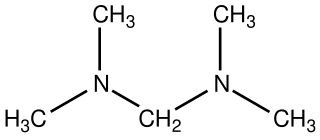
Bis(dimethylamino)methane is the organic compound with the formula [(CH3)2N]2CH2. It is classified as an aminal as well as a ditertiary amine, in fact the simplest. It is a colorless liquid that is widely available. It is prepared by the reaction of dimethylamine and formaldehyde: