CD32, also known as FcγRII or FCGR2, is a surface receptor glycoprotein belonging to the Ig gene superfamily. CD32 can be found on the surface of a variety of immune cells. CD32 has a low-affinity for the Fc region of IgG antibodies in monomeric form, but high affinity for IgG immune complexes. CD32 has two major functions: cellular response regulation, and the uptake of immune complexes. Cellular responses regulated by CD32 include phagocytosis, cytokine stimulation, and endocytic transport. Dysregulated CD32 is associated with different forms of autoimmunity, including systemic lupus erythematosus. In humans, there are three major CD32 subtypes: CD32A, CD32B, and CD32C. While CD32A and CD32C are involved in activating cellular responses, CD32B is inhibitory.

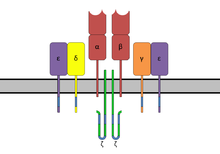
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.

In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.
Lck is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK), it is important for the activation of the T-cell receptor signaling in both naive T cells and effector T cells. The role of the Lck is less prominent in the activation or in the maintenance of memory CD8 T cells in comparison to CD4 T cells. In addition, the role of the lck varies among the memory T cells subsets. It seems that in mice, in the effector memory T cells (TEM) population, more than 50% of lck is present in a constitutively active conformation, whereas, only less than 20% of lck is present as active form of lck. These differences are due to differential regulation by SH2 domain–containing phosphatase-1 (Shp-1) and C-terminal Src kinase.

ZAP-70 is a protein normally expressed near the surface membrane of lymphocytes. It is most prominently known to be recruited upon antigen binding to the T cell receptor (TCR), and it plays a critical role in T cell signaling.

CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.
An immunoreceptor tyrosine-based inhibitory motif (ITIM), is a conserved sequence of amino acids that is found intracellularly in the cytoplasmic domains of many inhibitory receptors of the non-catalytic tyrosine-phosphorylated receptor family found on immune cells. These immune cells include T cells, B cells, NK cells, dendritic cells, macrophages and mast cells. ITIMs have similar structures of S/I/V/LxYxxI/V/L, where x is any amino acid, Y is a tyrosine residue that can be phosphorylated, S is the amino acid serine, I is the amino acid isoleucine, and V is the amino acid valine. ITIMs recruit SH2 domain-containing phosphatases, which inhibit cellular activation. ITIM-containing receptors often serve to target immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors, resulting in an innate inhibition mechanism within cells. ITIM bearing receptors have important role in regulation of immune system allowing negative regulation at different levels of the immune response.
Ly49 is a family of membrane C-type lectin-like receptors expressed mainly on NK cells but also on other immune cells. Their primary role is to bind MHC-I molecules to distinguish between self healthy cells and infected or altered cells. Ly49 family is coded by Klra gene cluster and include genes for both inhibitory and activating paired receptors, but most of them are inhibitory. Inhibitory Ly49 receptors play a role in the recognition of self cells and thus maintain self-tolerance and prevent autoimmunity by suppressing NK cell activation. On the other hand, activating receptors recognise ligands from cancer or viral infected cells and are used when cells lack or have abnormal expression of MHC-I molecules, which activate cytokine production and cytotoxic activity of NK and immune cells.
Tyrosine-protein kinase Lyn is a protein that in humans is encoded by the LYN gene.

TYRO protein tyrosine kinase-binding protein is an adapter protein that in humans is encoded by the TYROBP gene.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.

Fc receptor-like protein 3 is a protein that in humans is encoded by the FCRL3 gene.

Cluster of differentiation CD79A also known as B-cell antigen receptor complex-associated protein alpha chain and MB-1 membrane glycoprotein, is a protein that in humans is encoded by the CD79A gene.

Fc fragment of IgA receptor (FCAR) is a human gene that codes for the transmembrane receptor FcαRI, also known as CD89. FcαRI binds the heavy-chain constant region of Immunoglobulin A (IgA) antibodies. FcαRI is present on the cell surface of myeloid lineage cells, including neutrophils, monocytes, macrophages, and eosinophils, though it is notably absent from intestinal macrophages and does not appear on mast cells. FcαRI plays a role in both pro- and anti-inflammatory responses depending on the state of IgA bound. Inside-out signaling primes FcαRI in order for it to bind its ligand, while outside-in signaling caused by ligand binding depends on FcαRI association with the Fc receptor gamma chain.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.
Epstein–Barr virus (EBV) latent membrane protein 2 (LMP2) are two viral proteins of the Epstein–Barr virus. LMP2A/LMP2B are transmembrane proteins that act to block tyrosine kinase signaling. LMP2A is a transmembrane protein that inhibits normal B-cell signal transduction by mimicking an activated B-cell receptor (BCR). The N-terminus domain of LMP2A is tyrosine phosphorylated and associates with Src family protein tyrosine kinases (PTKs) as well as spleen tyrosine kinase (Syk). PTKs and Syk are associated with BCR signal transduction.

Killer Activation Receptors (KARs) are receptors expressed on the plasmatic membrane of Natural Killer cells. KARs work together with inhibitory receptors, which inactivate them in order to regulate the NK cells functions on hosted or transformed cells. These two kinds of specific receptors have some morphological features in common, such as being transmembrane proteins. The similarities are specially found in the extracellular domains and, the differences tend to be in the intracellular domains. KARs and KIRs can have tyrosine containing activatory or inhibitory motifs in the intracellular part of the receptor molecule.
CD94/NKG2 is a family of C-type lectin receptors which are expressed predominantly on the surface of NK cells and a subset of CD8+ T-lymphocyte. These receptors stimulate or inhibit cytotoxic activity of NK cells, therefore they are divided into activating and inhibitory receptors according to their function. CD94/NKG2 recognize nonclassical MHC glycoproteins class I (HLA-E in human and Qa-1 molecules in the mouse).
Non-catalytic tyrosine-phosphorylated receptors (NTRs), also called immunoreceptors or Src-family kinase-dependent receptors, are a group of cell surface receptors expressed by leukocytes that are important for cell migration and the recognition of abnormal cells or structures and the initiation of an immune response. These transmembrane receptors are not grouped into the NTR family based on sequence homology, but because they share a conserved signalling pathway utilizing the same signalling motifs. A signaling cascade is initiated when the receptors bind their respective ligand resulting in cell activation. For that tyrosine residues in the cytoplasmic tail of the receptors have to be phosphorylated, hence the receptors are referred to as tyrosine-phosphorylated receptors. They are called non-catalytic receptors, as the receptors have no intrinsic tyrosine kinase activity and cannot phosphorylate their own tyrosine residues. Phosphorylation is mediated by additionally recruited kinases. A prominent member of this receptor family is the T-cell receptor.

Dectin-2 or C-type lectin domain containing 6A is a protein that in humans is encoded by the CLEC6A gene. Dectin-2 is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded protein is a type II transmembrane protein with an extracellular carbohydrate recognition domain. It functions as a pattern recognition receptor recognizing α-mannans and as such plays an important role in innate immune response to fungi. Expression is found on macrophages and dendritic cells. It can also be found at low levels in Langerhans cells and peripheral blood monocytes, where expression levels could be increased upon induction of inflammation.