
Agnosia is the inability to process sensory information. Often there is a loss of ability to recognize objects, persons, sounds, shapes, or smells while the specific sense is not defective nor is there any significant memory loss. It is usually associated with brain injury or neurological illness, particularly after damage to the occipitotemporal border, which is part of the ventral stream. Agnosia only affects a single modality, such as vision or hearing. More recently, a top-down interruption is considered to cause the disturbance of handling perceptual information.

The visual system comprises the sensory organ and parts of the central nervous system which gives organisms the sense of sight as well as enabling the formation of several non-image photo response functions. It detects and interprets information from the optical spectrum perceptible to that species to "build a representation" of the surrounding environment. The visual system carries out a number of complex tasks, including the reception of light and the formation of monocular neural representations, colour vision, the neural mechanisms underlying stereopsis and assessment of distances to and between objects, the identification of particular object of interest, motion perception, the analysis and integration of visual information, pattern recognition, accurate motor coordination under visual guidance, and more. The neuropsychological side of visual information processing is known as visual perception, an abnormality of which is called visual impairment, and a complete absence of which is called blindness. Non-image forming visual functions, independent of visual perception, include the pupillary light reflex (PLR) and circadian photoentrainment.

The occipital lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The name derives from its position at the back of the head, from the Latin ob, behind, and caput, the head.

Prosopagnosia, also called face blindness, is a cognitive disorder of face perception in which the ability to recognize familiar faces, including one's own face (self-recognition), is impaired, while other aspects of visual processing and intellectual functioning remain intact. The term originally referred to a condition following acute brain damage, but a congenital or developmental form of the disorder also exists, with a prevalence rate of 2.5%. The specific brain area usually associated with prosopagnosia is the fusiform gyrus, which activates specifically in response to faces. The functionality of the fusiform gyrus allows most people to recognize faces in more detail than they do similarly complex inanimate objects. For those with prosopagnosia, the new method for recognizing faces depends on the less sensitive object-recognition system. The right hemisphere fusiform gyrus is more often involved in familiar face recognition than the left. It remains unclear whether the fusiform gyrus is only specific for the recognition of human faces or if it is also involved in highly trained visual stimuli.

The cerebrum, telencephalon or endbrain, is the largest part of the brain containing the cerebral cortex, as well as several subcortical structures, including the hippocampus, basal ganglia, and olfactory bulb. In the human brain, the cerebrum is the uppermost region of the central nervous system. The cerebrum develops prenatally from the forebrain (prosencephalon). In mammals, the dorsal telencephalon, or pallium, develops into the cerebral cortex, and the ventral telencephalon, or subpallium, becomes the basal ganglia. The cerebrum is also divided into approximately symmetric left and right cerebral hemispheres.
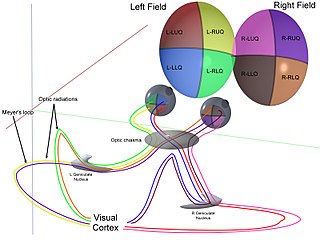
The optic radiation are axons from the neurons in the lateral geniculate nucleus to the primary visual cortex. The optic radiation receives blood through deep branches of the middle cerebral artery and posterior cerebral artery.

The fusiform gyrus, also known as the lateral occipitotemporal gyrus,is part of the temporal lobe and occipital lobe in Brodmann area 37. The fusiform gyrus is located between the lingual gyrus and parahippocampal gyrus above, and the inferior temporal gyrus below. Though the functionality of the fusiform gyrus is not fully understood, it has been linked with various neural pathways related to recognition. Additionally, it has been linked to various neurological phenomena such as synesthesia, dyslexia, and prosopagnosia.

The arcuate fasciculus (AF) is a bundle of axons that generally connects the Broca's area and the Wernicke's area in the brain. It is an association fiber tract connecting caudal temporal cortex and inferior frontal lobe. Fasciculus arcuatus is latin for curved bundle.

The inferior temporal gyrus is one of three gyri of the temporal lobe and is located below the middle temporal gyrus, connected behind with the inferior occipital gyrus; it also extends around the infero-lateral border on to the inferior surface of the temporal lobe, where it is limited by the inferior sulcus. This region is one of the higher levels of the ventral stream of visual processing, associated with the representation of objects, places, faces, and colors. It may also be involved in face perception, and in the recognition of numbers.

The superior parietal lobule is bounded in front by the upper part of the postcentral sulcus, but is usually connected with the postcentral gyrus above the end of the sulcus. The superior parietal lobule contains Brodmann's areas 5 and 7.

The commissural fibers or transverse fibers are axons that connect the two hemispheres of the brain. In contrast to commissural fibers, association fibers connect regions within the same hemisphere of the brain, and projection fibers connect each region to other parts of the brain or to the spinal cord.
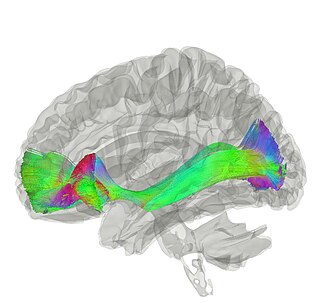
The occipitofrontal fasciculus, also known as the fronto-occipital fasciculus, passes backward from the frontal lobe, along the lateral border of the caudate nucleus, and on the medial aspect of the corona radiata; its fibers radiate in a fan-like manner and pass into the occipital and temporal lobes lateral to the posterior and inferior cornua.

The uncinate fasciculus is a white matter association tract in the human brain that connects parts of the limbic system such as the temporal pole, anterior parahippocampus, and amygdala in the temporal lobe with inferior portions of the frontal lobe such as the orbitofrontal cortex. Its function is unknown though it is affected in several psychiatric conditions. It is one of the last white matter tracts to mature in the human brain.

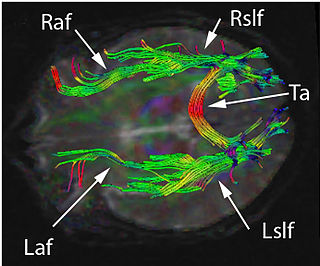
The superior longitudinal fasciculus (SLF) is an association fiber tract in the brain that is composed of three separate components. It is present in both hemispheres and can be found lateral to the centrum semiovale and connects the frontal, occipital, parietal, and temporal lobes. These bundles of axon tracts pass from the frontal lobe through the operculum to the posterior end of the lateral sulcus where they either radiate to and synapse on neurons in the occipital lobe or turn downward and forward around the putamen and then radiate to and synapse on neurons in anterior portions of the temporal lobe.
Pure alexia, also known as agnosic alexia or alexia without agraphia or pure word blindness, is one form of alexia which makes up "the peripheral dyslexia" group. Individuals who have pure alexia have severe reading problems while other language-related skills such as naming, oral repetition, auditory comprehension or writing are typically intact.
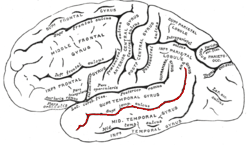
The superior temporal sulcus (STS) is the sulcus separating the superior temporal gyrus from the middle temporal gyrus in the temporal lobe of the brain. A sulcus is a deep groove that curves into the largest part of the brain, the cerebrum, and a gyrus is the a ridge that curves outward of the cerebrum.
Phonagnosia is a type of agnosia, or loss of knowledge, that involves a disturbance in the recognition of familiar voices and the impairment of voice discrimination abilities in which the affected individual does not suffer from comprehension deficits. Phonagnosia is an auditory agnosia, an acquired auditory processing disorder resulting from brain damage, other auditory agnosias include cortical deafness and auditory verbal agnosia also known as pure word deafness.
Disconnection syndrome is a general term for a collection of neurological symptoms caused -- via lesions to associational or commissural nerve fibres -- by damage to the white matter axons of communication pathways in the cerebrum, independent of any lesions to the cortex. The behavioral effects of such disconnections are relatively predictable in adults. Disconnection syndromes usually reflect circumstances where regions A and B still have their functional specializations except in domains that depend on the interconnections between the two regions.
The parieto-frontal integration theory (P-FIT) considers intelligence to relate to how well different brain regions integrate to form intelligent behaviors. The theory proposes that large scale brain networks connect brain regions, including regions within frontal, parietal, temporal, and cingulate cortices, underlie the biological basis of human intelligence. These regions, which overlap significantly with the task-positive network, allow the brain to communicate and exchange information efficiently with one another. Support for this theory is primarily based on neuroimaging evidence, with support from lesion studies. The P-FIT is influential in that it explains the majority of current neuroimaging findings, as well as increasing empirical support for cognition being the result of large-scale brain networks, rather than numerous domain-specific processes or modules. A 2010 review of the neuroscience of intelligence described P-FIT as "the best available answer to the question of where in the brain intelligence resides".
















