Helium-3 is a light, stable isotope of helium with two protons and one neutron. Other than protium, helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939.
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares the abundance of a naturally occurring radioactive isotope within the material to the abundance of its decay products, which form at a known constant rate of decay. The use of radiometric dating was first published in 1907 by Bertram Boltwood and is now the principal source of information about the absolute age of rocks and other geological features, including the age of fossilized life forms or the age of Earth itself, and can also be used to date a wide range of natural and man-made materials.
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System, and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt. It is an integrated field of chemistry and geology.
In physical cosmology, Big Bang nucleosynthesis is the production of nuclei other than those of the lightest isotope of hydrogen during the early phases of the universe. This type of nucleosynthesis is thought by most cosmologists to have occurred from 10 seconds to 20 minutes after the Big Bang. It is thought to be responsible for the formation of most of the universe's helium, along with small fractions of the hydrogen isotope deuterium, the helium isotope helium-3 (3He), and a very small fraction of the lithium isotope lithium-7 (7Li). In addition to these stable nuclei, two unstable or radioactive isotopes were produced: the heavy hydrogen isotope tritium and the beryllium isotope beryllium-7 (7Be). These unstable isotopes later decayed into 3He and 7Li, respectively, as above.

A mantle plume is a proposed mechanism of convection within the Earth's mantle, hypothesized to explain anomalous volcanism. Because the plume head partially melts on reaching shallow depths, a plume is often invoked as the cause of volcanic hotspots, such as Hawaii or Iceland, and large igneous provinces such as the Deccan and Siberian Traps. Some such volcanic regions lie far from tectonic plate boundaries, while others represent unusually large-volume volcanism near plate boundaries.
Isotopic labeling is a technique used to track the passage of an isotope through chemical reaction, metabolic pathway, or a biological cell. The reactant is 'labeled' by replacing one or more specific atoms with their isotopes. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.
Osmium (76Os) has seven naturally occurring isotopes, five of which are stable: 187Os, 188Os, 189Os, 190Os, and (most abundant) 192Os. The other natural isotopes, 184Os, and 186Os, have extremely long half-life (1.12×1013 years and 2×1015 years, respectively) and for practical purposes can be considered to be stable as well. 187Os is the daughter of 187Re (half-life 4.56×1010 years) and is most often measured in an 187Os/188Os ratio. This ratio, as well as the 187Re/188Os ratio, have been used extensively in dating terrestrial as well as meteoric rocks. It has also been used to measure the intensity of continental weathering over geologic time and to fix minimum ages for stabilization of the mantle roots of continental cratons. However, the most notable application of Os in dating has been in conjunction with iridium, to analyze the layer of shocked quartz along the Cretaceous–Paleogene boundary that marks the extinction of the dinosaurs 66 million years ago.
Samarium–neodymium dating is a radiometric dating method useful for determining the ages of rocks and meteorites, based on the alpha decay of the long-lived samarium isotope to the stable radiogenic neodymium isotope. Neodymium isotope ratios together with samarium-neodymium ratios are used to provide information on the age and source of igneous melts. It is sometimes assumed that at the moment when crustal material is formed from the mantle the neodymium isotope ratio depends only on the time when this event occurred, but thereafter it evolves in a way that depends on the new ratio of samarium to neodymium in the crustal material, which will be different from the ratio in the mantle material. Samarium–neodymium dating allows us to determine when the crustal material was formed.
Paleoceanography is the study of the history of the oceans in the geologic past with regard to circulation, chemistry, biology, geology and patterns of sedimentation and biological productivity. Paleoceanographic studies using environment models and different proxies enable the scientific community to assess the role of the oceanic processes in the global climate by the re-construction of past climate at various intervals. Paleoceanographic research is also intimately tied to paleoclimatology.

Isotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample.
The environmental isotopes are a subset of isotopes, both stable and radioactive, which are the object of isotope geochemistry. They are primarily used as tracers to see how things move around within the ocean-atmosphere system, within terrestrial biomes, within the Earth's surface, and between these broad domains.
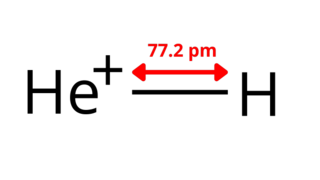
The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.
Rhenium–osmium dating is a form of radiometric dating based on the beta decay of the isotope 187Re to 187Os. This normally occurs with a half-life of 41.6 × 109 y, but studies using fully ionised 187Re atoms have found that this can decrease to only 33 y. Both rhenium and osmium are strongly siderophilic (iron loving), while Re is also chalcophilic (sulfur loving) making it useful in dating sulfide ores such as gold and Cu–Ni deposits.
Helium dating may refer to the traditional uranium–thorium dating or to a variety of He diffusion methods that utilize the mobility of He atoms to determine the thermal history of a rock. Helium diffusion experiments are often used to help interpret information retrieved from U–Th/He thermochronometric experiments. Kinematic parameters derived from He diffusion is done through estimating He diffusion over a range of temperatures. The use of density functional theory helps in estimating energy barriers for He to overcome as it diffuses across various crystallographic directions. Discrepancies, however, between observed and predicted He diffusion rates is still a problem and likely stem from unresolved problems in crystal defects and radiation damage in natural grains as opposed to theoretical grains. Depending on the mineral analyzed there are different assumptions to be made on He mobility. For example, He diffusion in minerals such as zircon, rutile, and monazite have been shown to be strongly anisotropic.
Harmon Craig was an American geochemist who worked briefly for the University of Chicago (1951-1955) before spending the majority of his career at Scripps Institution of Oceanography (1955-2003).

Ocean island basalt (OIB) is a volcanic rock, usually basaltic in composition, erupted in oceans away from tectonic plate boundaries. Although ocean island basaltic magma is mainly erupted as basalt lava, the basaltic magma is sometimes modified by igneous differentiation to produce a range of other volcanic rock types, for example, rhyolite in Iceland, and phonolite and trachyte at the intraplate volcano Fernando de Noronha. Unlike mid-ocean ridge basalts (MORBs), which erupt at spreading centers (divergent plate boundaries), and volcanic arc lavas, which erupt at subduction zones (convergent plate boundaries), ocean island basalts are the result of intraplate volcanism. However, some ocean island basalt locations coincide with plate boundaries like Iceland, which sits on top of a mid-ocean ridge, and Samoa, which is located near a subduction zone.

A radiogenic nuclide is a nuclide that is produced by a process of radioactive decay. It may itself be radioactive or stable.
The δ34S value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur, 34S:32S, in a sample against the equivalent ratio in a known reference standard. Presently, the most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil or per mille, using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfides, have been studied and interpreted as records of the changing sulfur cycle throughout the earth's history.
Isotopic reference materials are compounds with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in part, due to historical legacy, isotopic reference materials define the scales on which isotope ratios are reported in the peer-reviewed scientific literature.
Keiko Hattori is a geochemist and mineralogist. She is Distinguished University Professor of Geochemistry and Mineral Deposits in the Department of Earth and Environmental Sciences at the University of Ottawa.





