In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.
An isocyanide is an organic compound with the functional group –N+≡C−. It is the isomer of the related nitrile (–C≡N), hence the prefix is isocyano. The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

In organic chemistry, episulfides are a class of organic compounds that contain a saturated, heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes. Episulfides are less common and generally less stable than epoxides. The most common derivative is ethylene sulfide.

tert-Butyllithium is a chemical compound with the formula (CH3)3CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory.
In organic chemistry, a nitrone is a functional group consisting of an N-oxide of an imine. The general structure is R2C=N+O−R’, where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal [3+3] cycloadditions to form 6-membered rings, as well as formal [5+2] cycloadditions to form 7-membered rings.
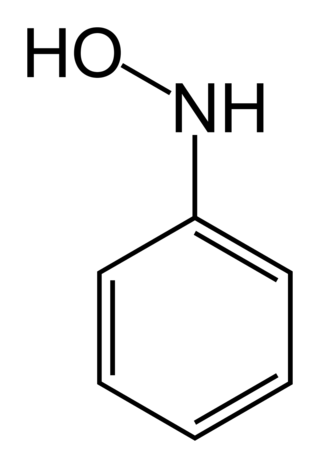
Phenylhydroxylamine is the organic compound with the formula C6H5NHOH. It is an intermediate in the redox-related pair C6H5NH2 and C6H5NO. Phenylhydroxylamine should not be confused with its isomer α-phenylhydroxylamine or O-phenylhydroxylamine.
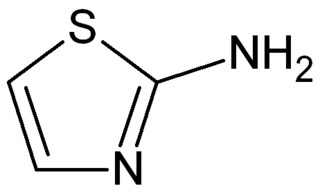
2-Aminothiazole is a heterocyclic amine featuring a thiazole core. It can also be considered a cyclic isothiourea. It possesses an odor similar to pyridine and is soluble in water, alcohols and diethyl ether. 2-Aminothiazole itself is mainly of academic interest, with few exceptions. It is a precursor to a sulfathiazole. 2-Aminothiazole can be used as a thyroid inhibitor in the treatment of hyperthyroidism.

Rolf Huisgen was a German chemist. His importance in synthetic organic chemistry extends to the enormous influence he had in post-war chemistry departments in Germany and Austria, due to a large number of his habilitants becoming professors. His major achievement was the development of the 1,3-dipolar cycloaddition reaction, also called the Huisgen cycloaddition.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
The nitrone-olefin [3+2] cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a [3+2] cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile.
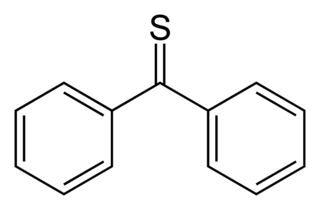
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.
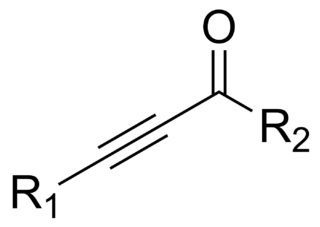
In organic chemistry, an ynone is an organic compound containing a ketone functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.
Isoxazolines are a class of five-membered heterocyclic chemical compounds, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line with the Hantzsch–Widman nomenclature. They are structural isomers of the more common oxazolines and exist in three different isomers depending on the location of the double bond. The relatively weak N-O bond makes isoxazolines prone to ring-opening and rearrangement reactions.

Chloroacetonitrile is the organic compound with the formula ClCH2CN. A colorless liquid, it is derived from acetonitrile (CH3CN) by replacement of one H with Cl. In practice, it is produced by dehydration of chloroacetamide. The compound is an alkylating agent, and as such is handled cautiously.
4-Chlorobutyronitrile is the organic compound with the formula ClCH2CH2CH2CN. With both chloro and cyano functional groups, it is a bifunctional molecule. It is a colorless liquid.