
An algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in freshwater or marine water systems. It is often recognized by the discoloration in the water from the algae's pigments. The term algae encompasses many types of aquatic photosynthetic organisms, both macroscopic multicellular organisms like seaweed and microscopic unicellular organisms like cyanobacteria. Algal bloom commonly refers to the rapid growth of microscopic unicellular algae, not macroscopic algae. An example of a macroscopic algal bloom is a kelp forest.
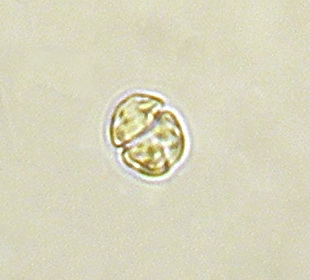
Gymnodinium is a genus of dinoflagellates, a type of marine and freshwater plankton. It is one of the few naked dinoflagellates, or species lacking armor known as cellulosic plates. Since 2000, the species which had been considered to be part of Gymnodinium have been divided into several genera, based on the nature of the apical groove and partial LSU rDNA sequence data. Amphidinium was redefined later. Gymnodinium belong to red dinoflagellates that, in concentration, can cause red tides. The red tides produced by some Gymnodinium, such as Gymnodinium catenatum, are toxic and pose risks to marine and human life, including paralytic shellfish poisoning.

Paralytic shellfish poisoning (PSP) is one of the four recognized syndromes of shellfish poisoning, which share some common features and are primarily associated with bivalve mollusks. These shellfish are filter feeders and accumulate neurotoxins, chiefly saxitoxin, produced by microscopic algae, such as dinoflagellates, diatoms, and cyanobacteria. Dinoflagellates of the genus Alexandrium are the most numerous and widespread saxitoxin producers and are responsible for PSP blooms in subarctic, temperate, and tropical locations. The majority of toxic blooms have been caused by the morphospecies Alexandrium catenella, Alexandrium tamarense, Gonyaulax catenella and Alexandrium fundyense, which together comprise the A. tamarense species complex. In Asia, PSP is mostly associated with the occurrence of the species Pyrodinium bahamense.

Thin layers are concentrated aggregations of phytoplankton and zooplankton in coastal and offshore waters that are vertically compressed to thicknesses ranging from several centimeters up to a few meters and are horizontally extensive, sometimes for kilometers. Generally, thin layers have three basic criteria: 1) they must be horizontally and temporally persistent; 2) they must not exceed a critical threshold of vertical thickness; and 3) they must exceed a critical threshold of maximum concentration. The precise values for critical thresholds of thin layers has been debated for a long time due to the vast diversity of plankton, instrumentation, and environmental conditions. Thin layers have distinct biological, chemical, optical, and acoustical signatures which are difficult to measure with traditional sampling techniques such as nets and bottles. However, there has been a surge in studies of thin layers within the past two decades due to major advances in technology and instrumentation. Phytoplankton are often measured by optical instruments that can detect fluorescence such as LIDAR, and zooplankton are often measured by acoustic instruments that can detect acoustic backscattering such as ABS. These extraordinary concentrations of plankton have important implications for many aspects of marine ecology, as well as for ocean optics and acoustics. Zooplankton thin layers are often found slightly under phytoplankton layers because many feed on them. Thin layers occur in a wide variety of ocean environments, including estuaries, coastal shelves, fjords, bays, and the open ocean, and they are often associated with some form of vertical structure in the water column, such as pycnoclines, and in zones of reduced flow.

Brevetoxin (PbTx), or brevetoxins, are a suite of cyclic polyether compounds produced naturally by a species of dinoflagellate known as Karenia brevis. Brevetoxins are neurotoxins that bind to voltage-gated sodium channels in nerve cells, leading to disruption of normal neurological processes and causing the illness clinically described as neurotoxic shellfish poisoning (NSP).

Neurotoxic shellfish poisoning (NSP) is caused by the consumption of brevetoxins, which are marine toxins produced by the dinoflagellate Karenia brevis. These toxins can produce a series of gastrointestinal and neurological effects. Outbreaks of NSP commonly take place following harmful algal bloom (HAB) events, commonly referred to as "Florida red tide". Algal blooms are a naturally-occurring phenomenon, however their frequency has been increasing in recent decades at least in-part due to human activities, climate changes, and the eutrophication of marine waters. HABs have been occurring for all of documented history, evidenced by the Native Americans' understanding of the dangers of shellfish consumption during periods of marine bioluminescence. Blooms have been noted to occur as far north as North Carolina and are commonly seen alongside the widespread death of fish and sea birds. In addition to the effects on human health, the economic impact of HAB-associated shellfish toxin outbreaks can have significant economic implications as well due to not only the associated healthcare costs, but the adverse impact on the commercial shellfish industry.

A harmful algal bloom (HAB), or excessive algae growth, is an algal bloom that causes negative impacts to other organisms by production of natural algae-produced toxins, mechanical damage to other organisms, or by other means. HABs are sometimes defined as only those algal blooms that produce toxins, and sometimes as any algal bloom that can result in severely lower oxygen levels in natural waters, killing organisms in marine or fresh waters. Blooms can last from a few days to many months. After the bloom dies, the microbes that decompose the dead algae use up more of the oxygen, generating a "dead zone" which can cause fish die-offs. When these zones cover a large area for an extended period of time, neither fish nor plants are able to survive. Harmful algal blooms in marine environments are often called "red tides".

Karenia is a genus that consists of unicellular, photosynthetic, planktonic organisms found in marine environments. The genus currently consists of 12 described species. They are best known for their dense toxic algal blooms and red tides that cause considerable ecological and economical damage; some Karenia species cause severe animal mortality. One species, Karenia brevis, is known to cause respiratory distress and neurotoxic shellfish poisoning (NSP) in humans.

The Gymnodiniales are an order of dinoflagellates, of the class Dinophyceae. Members of the order are known as gymnodinioid or gymnodinoid. They are athecate, or lacking an armored exterior, and as a result are relatively difficult to study because specimens are easily damaged. Many species are part of the marine plankton and are of interest primarily due to being found in algal blooms. As a group the gymnodinioids have been described as "likely one of the least known groups of the open ocean phytoplankton."

Akashiwo sanguinea is a species of marine dinoflagellates well known for forming blooms that result in red tides. The organism is unarmored (naked). Therefore, it lacks a thick cellulose wall, the theca, common in other genera of dinoflagellates. Reproduction of the phytoplankton species is primarily asexual.
Alexandrium monilatum is a species of armored, photosynthetic, marine dinoflagellates. It produces toxins that, when present in high concentrations as "red tides", can kill fish and reduce growth rates of shellfish.
Karenia mikimotoi is a dinoflagellate species from the genus Karenia. Its first appearance was in Japan in 1935 and since then, it has appeared in other parts of the world such as the east coast of the United States, Norway, and the English Channel.
Alexandrium catenella is a species of dinoflagellates. It is among the group of Alexandrium species that produce toxins that cause paralytic shellfish poisoning, and is a cause of red tide. ‘’Alexandrium catenella’’ is observed in cold, coastal waters, generally at temperate latitudes. These organisms have been found in the west coast of North America, Japan, Australia, and parts of South Africa.

Cochlodinium polykrikoides is a species of red tide producing marine dinoflagellates known for causing fish kills around the world, and well known for fish kills in marine waters of Southeast Asia. C. polykrikoides has a wide geographic range, including North America, Central America, Western India, Southwestern Europe and Eastern Asia. Single cells of this species are ovoidal in shape, 30-50μm in length and 25-30μm in width.
Karenia selliformis is a species from the genus Karenia, which are dinoflagellates. It was first discovered in New Zealand. Karenia selliformis produces the highly toxic gymnodimine, and as such is a potentially harmful ocean dweller. Gymnodimine is a nicotinic acetylcholine receptor-blocking phycotoxin, a source of shellfish poisoning.

Dinoflagellates are eukaryotic plankton, existing in marine and freshwater environments. Previously, dinoflagellates had been grouped into two categories, phagotrophs and phototrophs. Mixotrophs, however include a combination of phagotrophy and phototrophy. Mixotrophic dinoflagellates are a sub-type of planktonic dinoflagellates and are part of the phylum Dinoflagellata. They are flagellated eukaryotes that combine photoautotrophy when light is available, and heterotrophy via phagocytosis. Dinoflagellates are one of the most diverse and numerous species of phytoplankton, second to diatoms.
Jan H. Landsberg is a biologist, researcher, and author. Her professional research interests in biology have particular focus on aquatic animal and environmental health.
Pseudo-nitzschia australis is a pennate diatom found in temperate and sub-tropic marine waters, such as off the coast of California and Argentina. This diatom is a Harmful Micro Algae that produces toxic effects on a variety of organisms through its production of domoic acid, a neurotoxin. Toxic effects have been observed in a variety of predatory organisms such as pelicans, sea lions, and humans. If exposed to a high enough dose, these predators will die as a result, and there is no known antidote. The potential indirect mortality associated with P. australis is of great concern to humans as toxic algae blooms, including blooms of P. australis, continue to increase in frequency and severity over recent years. Blooms of P. australis are believed to result from high concentrations of nitrates and phosphates in stream and river runoff, as well as coastal upwelling, which are also sources of other harmful algae blooms.
Lepidodinium is a genus of dinoflagellates belonging to the family Gymnodiniaceae. Lepidodinium is a genus of green dinoflagellates in the family Gymnodiniales. It contains two different species, Lepidodiniumchlorophorum and Lepidodinium viride. They are characterised by their green colour caused by a plastid derived from Pedinophyceae, a green algae group. This plastid has retained chlorophyll a and b, which is significant because it differs from the chlorophyll a and c usually observed in dinoflagellate peridinin plastids. They are the only known dinoflagellate genus to possess plastids derived from green algae. Lepidodinium chlorophorum is known to cause sea blooms, partially off the coast of France, which has dramatic ecological and economic consequences. Lepidodinium produces some of the highest volumes of Transparent Exopolymer Particles of any phytoplankton, which can contribute to bivalve death and the creation of anoxic conditions in blooms, as well as playing an important role in carbon cycling in the ocean.
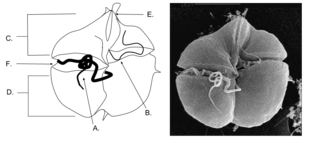
Kareniaceae is a accepted marine family of relatively small, toxic, unarmored dinoflagellates belonging to the order Gymnodiniales. Species in the Kareniaceae clade often times cause discolored green Harmful algea blooms (HABs) that pose a safety and health risk to humans and the regions around it. Such blooms also pose a risk to coastal aquaculture worldwide, especially places like France, Atlantic ocean, the English channel and the Mediterranean sea.












