The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

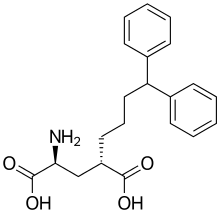
Eglumetad is a research drug developed by Eli Lilly and Company, which is being investigated for its potential in the treatment of anxiety and drug addiction. It is a glutamate derived compound and its mode of action implies a novel mechanism.
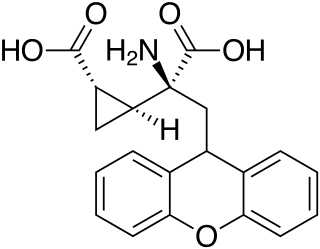
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

EGLU is a drug that is used in neuroscience research. It was one of the first compounds found that acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), and so has been useful in the characterization and study of this receptor subfamily.

LY-344,545 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as an antagonist for the metabotropic glutamate receptor subtype mGluR5. It is an epimer of another metabotropic glutamate receptor antagonist, the mGluR2/3-selective LY-341,495.

Pomaglumetad (LY-404,039) is an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor group II subtypes mGluR2 and mGluR3. Pharmacological research has focused on its potential antipsychotic and anxiolytic effects. Pomaglumetad is intended as a treatment for schizophrenia and other psychotic and anxiety disorders by modulating glutamatergic activity and reducing presynaptic release of glutamate at synapses in limbic and forebrain areas relevant to these disorders. Human studies investigating therapeutic use of pomaglumetad have focused on the prodrug LY-2140023, a methionine amide of pomaglumetad (also called pomaglumetad methionil) since pomaglumetad exhibits low oral absorption and bioavailability in humans.

SIB-1757 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anti-hyperalgesia effects in animals. SIB-1757 along with other mGluR5 antagonists has been shown to have neuroprotective and hepatoprotective effects, and it is also used to study the role of the mGluR5 receptor in brain development.

SIB-1893 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anticonvulsant and neuroprotective effects, and reduces glutamate release. It has also been found to act as a positive allosteric modulator of mGluR4.

DCG-IV is a research drug which acts as a group-selective agonist for the group II metabotropic glutamate receptors (mGluR2/3). It has potent neuroprotective and anticonvulsant effects in animal studies, as well as showing anti-Parkinsonian effects, but also impairs the formation of memories.

LY-379,268 is a drug that is used in neuroscience research, which acts as a potent and selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).

LY-487,379 is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It is used to study the structure and function of this receptor subtype, and LY-487,379 along with various other mGluR2/3 agonists and positive modulators are being investigated as possible antipsychotic and anxiolytic drugs.

CECXG (3'-ethyl-LY-341,495) is a research drug which acts as a potent and selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with reasonable selectivity for mGluR3. While it is some five times less potent than LY-341,495 at mGluR3, it has 38x higher affinity for mGluR3 over mGluR2, making it one of the few ligands available that is able to distinguish between these two closely related receptor subtypes.

PCCG-4 is a research drug which acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), with slight selectivity for mGluR2 although not sufficient to distinguish mGluR2 and mGluR3 responses from each other. It is used in research into the function of the group II metabotropic glutamate receptors.

MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3). It produces antidepressant and anxiolytic effects in animal studies, and has been shown to boost release of dopamine and serotonin in specific brain areas. Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutamatergic drug ketamine.

RO-4491533 is a drug developed by Hoffmann-La Roche which acts as a potent and selective negative allosteric modulator for group II of the metabotropic glutamate receptors (mGluR2/3), being equipotent at mGluR2 and mGluR3 but without activity at other mGluR subtypes. In animal studies, RO-4491533 produced antidepressant effects and reversed the effects of the mGluR2/3 agonist LY-379,268 with similar efficacy but slightly lower potency than the mGluR2/3 antagonist LY-341,495. A number of related compounds are known, with similar effects in vitro and a fairly well characterized structure-activity relationship.