
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
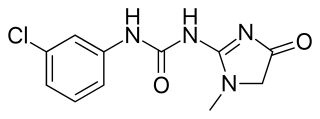
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

The glutamate receptor, metabotropic 1, also known as GRM1, is a human gene which encodes the metabotropic glutamate receptor 1 (mGluR1) protein.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

Metabotropic glutamate receptor 4 is a protein that in humans is encoded by the GRM4 gene.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

Metabotropic glutamate receptor 7 is a protein that in humans is encoded by the GRM7 gene.

Metabotropic glutamate receptor 8 is a protein that in humans is encoded by the GRM8 gene.
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Biphenylindanone A is a research agent which acts as a potent and selective positive allosteric modulator for the group II metabotropic glutamate receptor subtype mGluR2.

ADX-47273 is a research pharmaceutical developed by Addex Therapeutics which acts as a positive allosteric modulator (PAM) selective for the metabotropic glutamate receptor subtype mGluR5. It has nootropic and antipsychotic effects in animal studies, and has been used as a lead compound to develop improved derivatives.

SIB-1893 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anticonvulsant and neuroprotective effects, and reduces glutamate release. It has also been found to act as a positive allosteric modulator of mGluR4.

CDPPB is a drug used in scientific research which acts as a positive allosteric modulator selective for the metabotropic glutamate receptor subtype mGluR5. It has antipsychotic effects in animal models, and mGluR5 modulators are under investigation as potential drugs for the treatment of schizophrenia, as well as other applications.
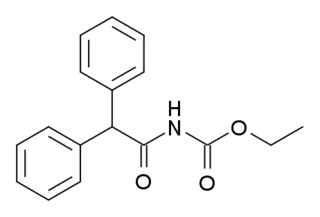
Ro01-6128 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of a lead compound found via high-throughput screening, and was further developed to give the improved compound Ro67-4853.

Ro67-4853 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of the simpler compound Ro01-6128, and has itself subsequently been used as a lead compound to develop a range of potent and selective mGluR1 positive modulators.

LY-487,379 is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It is used to study the structure and function of this receptor subtype, and LY-487,379 along with various other mGluR2/3 agonists and positive modulators are being investigated as possible antipsychotic and anxiolytic drugs.

VU-0238429 is a drug which acts as a selective positive allosteric modulator for the muscarinic acetylcholine receptor M5. It was the first selective ligand developed for the M5 subtype, and is structurally derived from older M1-selective positive allosteric modulators such as VU-0119498. Replacing the O-methyl- by a phenyl group further improves the receptor subtype selectivity.

CBiPES is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It has potentially antipsychotic effects in animal models, and is used for researching the role of mGluR2 receptors in schizophrenia and related disorders.
ADX-71149, also known as JNJ-40411813 and JNJ-mGluR2-PAM, is a selective positive allosteric modulator of the mGlu2 receptor. It is being studied by Addex Therapeutics and Janssen Pharmaceuticals for the treatment of schizophrenia. It was also researched by these companies for the treatment of anxious depression, but although some efficacy was observed in clinical trials, it was not enough to warrant further development for this indication. As of 2015, ADX-71149 is in phase II clinical trials for schizophrenia.

AZD 9272 is a drug which acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It was unsuccessful in human trials as an analgesic, but continues to be widely used in research especially as its radiolabelled forms.
























