
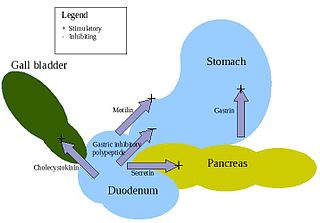
Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver. It is a peptide hormone produced in the S cells of the duodenum, which are located in the intestinal glands. In humans, the secretin peptide is encoded by the SCT gene.

Gastrin is a peptide hormone that stimulates secretion of gastric acid (HCl) by the parietal cells of the stomach and aids in gastric motility. It is released by G cells in the pyloric antrum of the stomach, duodenum, and the pancreas.

Ghrelin is a hormone produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid.
Glucose-dependent insulinotropic polypeptide (GIP), also known as Gastric inhibitory polypeptide or gastric inhibitory peptide, is an inhibiting hormone of the secretin family of hormones. While it is a weak inhibitor of gastric acid secretion, its main role is to stimulate insulin secretion.

Motilin is a 22-amino acid polypeptide hormone in the motilin family that, in humans, is encoded by the MLN gene.

Obestatin is a hormone that is produced in specialized epithelial cells of the stomach and small intestine of several animals including humans. Obestatin was originally identified as an anorectic peptide, but its effect on food intake remains controversial.

Urocortin is a protein that in humans is encoded by the UCN gene. Urocortin belongs to the corticotropin-releasing factor (CRF) family of proteins which includes CRF, urotensin I, sauvagine, urocortin II and urocortin III. Urocortin is involved in the mammalian stress response, and regulates aspects of appetite and stress response.

Peptide YY (PYY) also known as peptide tyrosine tyrosine is a peptide that in humans is encoded by the PYY gene. Peptide YY is a short peptide released from cells in the ileum and colon in response to feeding. In the blood, gut, and other elements of periphery, PYY acts to reduce appetite; similarly, when injected directly into the central nervous system, PYY is also anorexigenic, i.e., it reduces appetite.
The gastrointestinal hormones constitute a group of hormones secreted by enteroendocrine cells in the stomach, pancreas, and small intestine that control various functions of the digestive organs. Later studies showed that most of the gut peptides, such as secretin, cholecystokinin or substance P, were found to play a role of neurotransmitters and neuromodulators in the central and peripheral nervous systems.

The lateral hypothalamus (LH), also called the lateral hypothalamic area (LHA), contains the primary orexinergic nucleus within the hypothalamus that widely projects throughout the nervous system; this system of neurons mediates an array of cognitive and physical processes, such as promoting feeding behavior and arousal, reducing pain perception, and regulating body temperature, digestive functions, and blood pressure, among many others. Clinically significant disorders that involve dysfunctions of the orexinergic projection system include narcolepsy, motility disorders or functional gastrointestinal disorders involving visceral hypersensitivity, and eating disorders.
Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas. Microbiota plays key roles in the intestinal immune and metabolic responses in these enteroendocrine cells via their fermentation product, acetate.

Growth hormone secretagogue receptor(GHS-R), also known as ghrelin receptor, is a G protein-coupled receptor that binds growth hormone secretagogues (GHSs), such as ghrelin, the "hunger hormone". The role of GHS-R is thought to be in regulating energy homeostasis and body weight. In the brain, they are most highly expressed in the hypothalamus, specifically the ventromedial nucleus and arcuate nucleus. GSH-Rs are also expressed in other areas of the brain, including the ventral tegmental area, hippocampus, and substantia nigra. Outside the central nervous system, too, GSH-Rs are also found in the liver, in skeletal muscle, and even in the heart.

G-protein coupled receptor 39 is a protein that in humans is encoded by the GPR39 gene.

Galanin receptor 2, (GAL2) is a G-protein coupled receptor encoded by the GALR2 gene.

Melanin-concentrating hormone receptor 1, also known as MCH1, is one of the melanin-concentrating hormone receptors found in all mammals.

Melanin-concentrating hormone receptor 2 (MCH2) also known as G-protein coupled receptor 145 (GPR145) is a protein that in humans is encoded by the MCHR2 gene.

Itopride (INN; brand name Ganaton) is a prokinetic benzamide derivative. These drugs inhibit dopamine and acetylcholine esterase enzyme and have a gastrokinetic effect. Itopride is indicated for the treatment of functional dyspepsia and other gastrointestinal conditions. It is a combined D2 receptor antagonist and acetylcholinesterase inhibitor.

Ibutamoren (INN) is a potent, long-acting, orally-active, selective, and non-peptide agonist of the ghrelin receptor and a growth hormone secretagogue, mimicking the growth hormone (GH)-stimulating action of the endogenous hormone ghrelin. It has been shown to increase the secretion of several hormones including GH and insulin-like growth factor 1 (IGF-1) and produces sustained increases in the plasma levels of these hormones without affecting cortisol levels.
Relamorelin is a synthetic peptide, centrally penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) which is under development by Allergan pharmaceuticals for the treatment of diabetic gastroparesis, chronic idiopathic constipation, and anorexia nervosa. It is a pentapeptide and an analogue of ghrelin with improved potency and pharmacokinetics. In humans, relamorelin produces increases in plasma growth hormone, prolactin, and cortisol levels, and, like other GHSR agonists, increases appetite. As of June 2015, relamorelin is in phase II clinical trials for diabetic gastroparesis and constipation. The United States Food and Drug Administration (FDA) has granted Fast Track designation to relamorelin for diabetic gastroparesis.

Ulimorelin is a drug with a modified cyclic peptide structure which acts as a selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR-1a).. Unlike many related drugs, ulimorelin has little or no effect on growth hormone (GH) release in rats. However, like ghrelin and other ghrelin agonists, ulimorelin does stimulate GH release with concomitant increases in insulin-like growth factor 1 (IGF-1) in humans. It has been researched for enhancing gastrointestinal motility, especially in gastroparesis and in aiding recovery of bowel function following gastrointestinal surgery, where opioid analgesic drugs used for post-operative pain relief may worsen existing constipation. While ulimorelin has been shown to increase both upper and lower gastrointestinal motility in rats, and showed promising results initially in humans, it failed in pivotal clinical trials in post operative ileus.


















