Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting:
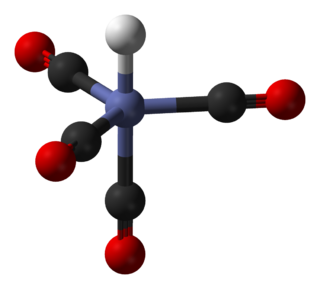
In coordination chemistry, a ligand is an ion or molecule that binds to a central atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs often through Lewis Bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Ferrocene is an organometallic compound with the formula Fe(C
5H
5)
2. The molecule consists of two cyclopentadienyl rings bound on opposite sides of a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C
5H
5)+
2.

A cyclopentadienyl complex is a metal complex with one or more cyclopentadienyl groups. Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (η5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.

The isolobal principle is a strategy used in organometallic chemistry to relate the structure of organic and inorganic molecular fragments in order to predict bonding properties of organometallic compounds. Roald Hoffmann described molecular fragments as isolobal "if the number, symmetry properties, approximate energy and shape of the frontier orbitals and the number of electrons in them are similar – not identical, but similar." One can predict the bonding and reactivity of a lesser-known species from that of a better-known species if the two molecular fragments have similar frontier orbitals, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Isolobal compounds are analogues to isoelectronic compounds that share the same number of valence electrons and structure. A graphic representation of isolobal structures, with the isolobal pairs connected through a double-headed arrow with half an orbital below, is found in Figure 1.
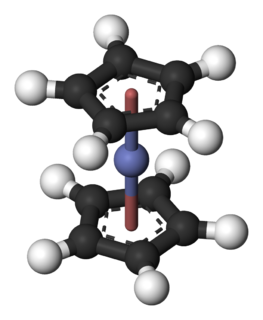
Cobaltocene, known also as bis(cyclopentadienyl)cobalt(II) or even "bis Cp cobalt", is an organocobalt compound with the formula Co(C5H5)2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene. Due to the ease with which it reacts with oxygen, the compound must be handled and stored using air-free techniques.

Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

Chromocene is the organochromium compound with the formula [Cr(C5H5)2]. Like structurally related metallocenes, chromocene readily sublimes in a vacuum and is soluble in non-polar organic solvents. It is more formally known as bis(η5-cyclopentadienyl)chromium(II).

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Organotitanium compounds in organometallic chemistry contain carbon-to-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.

Organouranium chemistry is the science exploring the properties, structure and reactivity of organouranium compounds, which are organometallic compounds containing a carbon to uranium chemical bond. The field is of some importance to the nuclear industry and of theoretical interest in organometallic chemistry.
In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related to the η5cyclopentadienyl anion (Cp), thus indenyl analogues of many cyclopentadienyl complexes are known. Indenyl ligands lack the 5-fold symmetry of Cp, so they exhibit more complicated geometries. Furthermore, some indenyl complexes also exist with only η3-bonding mode. The η5- and η3-bonding modes sometimes interconvert.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
Organoscandium chemistry is the chemistry of organometallic compounds containing a carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but several compound classes find practical application in catalysis, especially in polymerization. A common precursor is scandium chloride.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C or when trapped by cooling to liquid nitrogen temperatures (−196 °C). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.
The covalent bond classification (CBC) method is also referred to as the LXZ notation. It was published by M. L. H. Green in 1995 as a solution for the need to describe covalent compounds such as organometallic complexes in a way that is not prone to limitations resulting from the definition of oxidation state. Instead of simply assigning a charge to an atom in the molecule, the covalent bond classification method analyzes the nature of the ligands surrounding the atom of interest, which is often a transition metal. According to this method, there are three basic types of interactions that allow for coordination of the ligand. The three types of interaction are classified according to whether the ligating group donates two, one, or zero electrons. These three classes of ligands are respectively given the symbols L, X, and Z.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
Magnesocene, also known as bis(cyclopentadienyl)magnesium(II) and sometimes abbreviated as MgCp2, is an organometallic compound with the formula Mg(η5-C5H5)2. It is an example of an s-block main group sandwich compound, structurally related to the d-block element metallocenes, and consists of a central magnesium atom sandwiched between two cyclopentadienyl rings.






















