Antimycins are produced as secondary metabolites by Streptomyces bacteria, a soil bacteria. These specialized metabolites likely function to kill neighboring organisms in order to provide the streptomyces bacteria with a competitive edge.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.
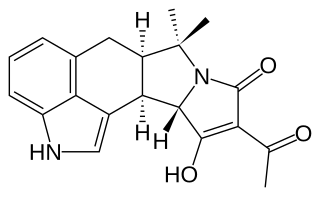
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.

Moorea producens is a species of filamentous cyanobacteria in the genus Moorea, including tropical marine strains formerly classified as Lyngbya majuscula due to morphological resemblance but separated based on genetic evidence. Moorea producens grows on seagrass and is one of the causes of the human skin irritation seaweed dermatitis. It is known as fireweed in Australia and stinging limu in Hawaii.

Staurosporine is a natural product originally isolated in 1977 from the bacterium Streptomyces staurosporeus. It was the first of over 50 alkaloids to be isolated with this type of bis-indole chemical structure. The chemical structure of staurosporine was elucidated by X-ray analysis of a single crystal and the absolute stereochemical configuration by the same method in 1994.
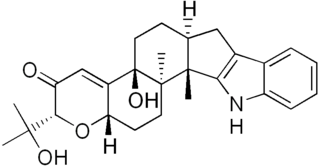
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by Penicillium paxilli which was first characterized in 1975. Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer as well as antimicrobial drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
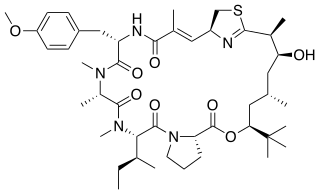
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.

Lyngbya majuscula is a species of filamentous cyanobacteria in the genus Lyngbya. It is named after the Dane Hans Christian Lyngbye.
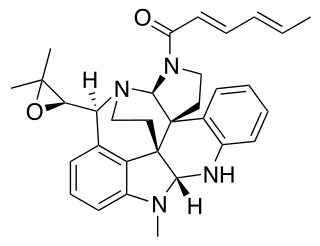
Communesin B is a cytotoxic chemical compound isolated from Penicillium strains found on the marine alga Ulva intestinalis. It exhibits cytotoxicity in vitro against human lung carcinoma, prostate carcinoma, colorectal carcinoma, cervical adenocarcinoma, and breast adenocarcinoma cell lines.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.

Nosiheptide is a thiopeptide antibiotic produced by the bacterium Streptomyces actuosus.
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.

Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.
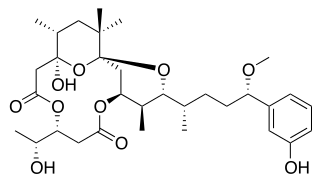
Debromoaplysiatoxin is a toxic agent produced by the blue-green alga Lyngbya majuscula. This alga lives in marine waters and causes seaweed dermatitis. Furthermore, it is a tumor promoter which has an anti-proliferative activity against various cancer cell lines in mice.

Pyonitrins are a family of highly hydrogen-deficient alkaloids discovered from an insect-associated Pseudomonas protegens strain. In vivo, pyonitrins A-D show activity against pathogen Candida albicans, which commonly cause bloodstream infections.
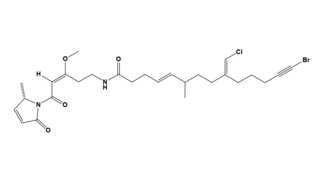
Jamaicamide A is a lipopeptide isolated from the cyanobacterium Moorea producens, formerly known as Lyngbya majuscula. Jamaicamide A belongs to a family of compounds collectively called jamaicamides, which are sodium channel blockers with potent neurotoxicity in a cellular model. Jamaicamide A has several unusual functionalities, including an alkynyl bromide, vinyl chloride, β-methoxy eneone system, and pyrrolinone ring.
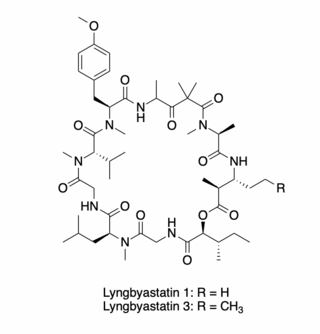
Lyngbyastatins 1 and 3 are cytotoxic cyclic depsipeptides that possess antiproliferative activity against human cancer cell lines. These compounds, first isolated from the extract of a Lyngbya majuscula/Schizothrix calcicola assemblage and from L. majuscula Harvey ex Gomont (Oscillatoriaceae) strains, respectively, target the actin cytoskeleton of eukaryotic cells.
















