Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG.

Tryptophan synthase or tryptophan synthetase is an enzyme that catalyzes the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animalia. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25 Ångstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channeling. The active sites of tryptophan synthase are allosterically coupled.

Cyanotoxins are toxins produced by cyanobacteria. Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under high concentration of phosphorus conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they can poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.

Hydrilla (waterthyme) is a genus of aquatic plant, usually treated as containing just one species, Hydrilla verticillata, though some botanists divide it into several species. It is native to the cool and warm waters of the Old World in Asia, Africa and Australia, with a sparse, scattered distribution; in Australia from Northern Territory, Queensland, and New South Wales.

Paralytic shellfish poisoning (PSP) is one of the four recognized syndromes of shellfish poisoning, which share some common features and are primarily associated with bivalve mollusks. These shellfish are filter feeders and accumulate neurotoxins, chiefly saxitoxin, produced by microscopic algae, such as dinoflagellates, diatoms, and cyanobacteria. Dinoflagellates of the genus Alexandrium are the most numerous and widespread saxitoxin producers and are responsible for PSP blooms in subarctic, temperate, and tropical locations. The majority of toxic blooms have been caused by the morphospecies Alexandrium catenella, Alexandrium tamarense, Gonyaulax catenella and Alexandrium fundyense, which together comprise the A. tamarense species complex. In Asia, PSP is mostly associated with the occurrence of the species Pyrodinium bahamense.
Cyclopiazonic acid (α-CPA), a mycotoxin and a fungal neurotoxin, is made by the molds Aspergillus and Penicillium. It is an indole-tetramic acid that serves as a toxin due to its ability to inhibit calcium-dependent ATPases found in the endoplasmic and sarcoplasmic reticulum. This inhibition disrupts the muscle contraction-relaxation cycle and the calcium gradient that is maintained for proper cellular activity in cells.
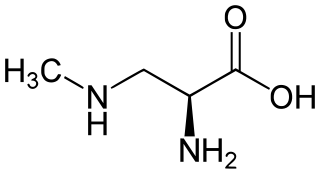
β-Methylamino-L-alanine, or BMAA, is a non-proteinogenic amino acid produced by cyanobacteria. BMAA is a neurotoxin. Its potential role in various neurodegenerative disorders is the subject of scientific research.

Staurosporine is a natural product originally isolated in 1977 from the bacterium Streptomyces staurosporeus. It was the first of over 50 alkaloids that were discovered to share this type of bis-indole chemical structure. The chemical structure of staurosporine was elucidated by X-ray crystalography in 1994.

Anatoxin-a, also known as Very Fast Death Factor (VFDF), is a secondary, bicyclic amine alkaloid and cyanotoxin with acute neurotoxicity. It was first discovered in the early 1960s in Canada, and was isolated in 1972. The toxin is produced by multiple genera of cyanobacteria and has been reported in North America, South America, Central America, Europe, Africa, Asia, and Oceania. Symptoms of anatoxin-a toxicity include loss of coordination, muscular fasciculations, convulsions and death by respiratory paralysis. Its mode of action is through the nicotinic acetylcholine receptor (nAchR) where it mimics the binding of the receptor's natural ligand, acetylcholine. As such, anatoxin-a has been used for medicinal purposes to investigate diseases characterized by low acetylcholine levels. Due to its high toxicity and potential presence in drinking water, anatoxin-a poses a threat to animals, including humans. While methods for detection and water treatment exist, scientists have called for more research to improve reliability and efficacy. Anatoxin-a is not to be confused with guanitoxin, another potent cyanotoxin that has a similar mechanism of action to that of anatoxin-a and is produced by many of the same cyanobacteria genera, but is structurally unrelated.

Nodularins are potent toxins produced by the cyanobacterium Nodularia spumigena, among others. This aquatic, photosynthetic cyanobacterium forms visible colonies that present as algal blooms in brackish water bodies throughout the world. The late summer blooms of Nodularia spumigena are among the largest cyanobacterial mass occurrences in the world. Cyanobacteria are composed of many toxic substances, most notably of microcystins and nodularins: the two are not easily differentiated. A significant homology of structure and function exists between the two, and microcystins have been studied in greater detail. Because of this, facts from microcystins are often extended to nodularins.
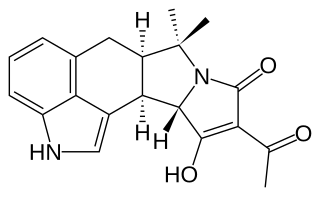
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by Penicillium paxilli which was first characterized in 1975. Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic.
AETX refers to a group of polypeptide neurotoxins isolated from the sea anemone Anemonia erythraea that target ion channels, altering their function. Four subtypes have been identified: AETX I, II, III and K, which vary in their structure and target.

Lyngbyatoxin-a is a cyanotoxin produced by certain cyanobacteria species, most notably Moorea producens. It is produced as defense mechanism to ward off any would-be predators of the bacterium, being a potent blister agent as well as carcinogen. Low concentrations cause a common skin condition known as seaweed dermatitis.

Scytonemin is a secondary metabolite and an extracellular matrix (sheath) pigment synthesized by many strains of cyanobacteria, including Nostoc, Scytonema, Calothrix, Lyngbya, Rivularia, Chlorogloeopsis, and Hyella. Scytonemin-synthesizing cyanobacteria often inhabit highly insolated terrestrial, freshwater and coastal environments such as deserts, semideserts, rocks, cliffs, marine intertidal flats, and hot springs.
Tryptophan N-monooxygenase (EC 1.14.13.125, tryptophan N-hydroxylase, CYP79B1, CYP79B2, CYP79B3) is an enzyme with systematic name L-tryptophan,NADPH:oxygen oxidoreductase (N-hydroxylating). This enzyme catalyses the following chemical reaction
Tryptophan 7-halogenase (EC 1.14.19.9, PrnA, RebH) is an enzyme with systematic name L-tryptophan:FADH2 oxidoreductase (7-halogenating). This enzyme catalyses the following chemical reaction:
L-tryptophan—pyruvate aminotransferase is an enzyme with systematic name L-tryptophan:pyruvate aminotransferase. This enzyme catalyses the following chemical reaction
Cyanophycin synthase (L-arginine-adding) is an enzyme with systematic name cyanophycin:L-arginine ligase (ADP-forming). This enzyme catalyses the following chemical reaction:
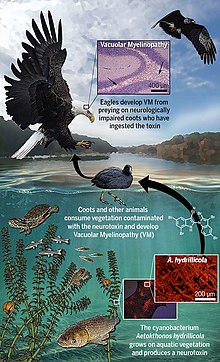
Avian vacuolar myelinopathy (AVM) is a fatal neurological disease that affects various waterbirds and raptors. It is most common in the bald eagle and American coot, and it is known in the killdeer, bufflehead, northern shoveler, American wigeon, Canada goose, great horned owl, mallard, and ring-necked duck. Avian vacuolar myelinopathy is a newly discovered disease that was first identified in the field in 1994 when dead bald eagles were found near DeGray Lake in Arkansas in the United States. Since then, it has spread to four more states and infested multiple aquatic systems including 10 reservoirs. The cause of death is lesions on the brain and spinal cord. A neurotoxin called aetokthonotoxin produced by cyanobacteria causes the disease.
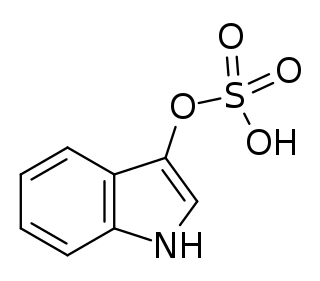
Indoxyl sulfate, also known as 3-indoxylsulfate and 3-indoxylsulfuric acid, is a metabolite of dietary L-tryptophan that acts as a cardiotoxin and uremic toxin. High concentrations of indoxyl sulfate in blood plasma are known to be associated with the development and progression of chronic kidney disease and vascular disease in humans. As a uremic toxin, it stimulates glomerular sclerosis and renal interstitial fibrosis.