Toxins targeting voltage-gated calcium channels (VGCC)
Huwentoxin-I
HWTX-I is the most abundant toxic component in the venom of H. schmidti. It inhibits presynaptic N-type Ca2+ channels.
Chemistry
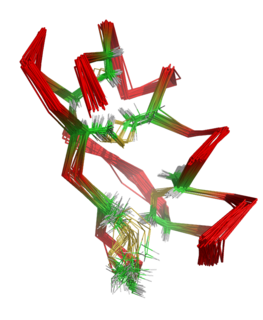
The molecular weight of HWTX-I is 3750 Da. The toxin comprises 33 residues, including six cysteines that form three disulfide linkages. [3] [4] These were assigned as Cys2-Cys17, Cys9-Cys22, and Cys16-Cys29 and are buried within the molecule. [5] The molecule adopts a compact structure consisting of a small triple-stranded antiparallel beta-sheet and five beta-turns. It was found that the structure contains an inhibitor cystine knot (ICK) motif. To form this motif, three disulfide bridges are needed. Two of them create a loop through which the third disulfide bridge passes. [4] [6] [7] The structure of HWTX-I is very stable, secondary structure elements do not significantly alter under different pH conditions or after heating. [8]
Mode of action
HWTX-I selectively inhibits N-type HVA channels. [9] A recent study found that HWTX-I also inhibits Na+ channels. [10]
Effects
In mice the intraperitoneal LD50 of HWTX-I is 0.70 mg/kg, the intracisternal LD50 has been determined as 9.40 µg/kg. Neurotoxic symptoms after intraperitoneal injection were gasping, excitation, spastic paralysis of the hindlimb and asynergia. [3] [11]
HWTX-I is a potential novel analgesic pharmaceutic. [12] Epidural administration of HWTX-I in rats with chronic neuropathic pain blocked heat hyperalgesia and mechanical allodynia in the injured hindpaw of rats, indicating that epidurally administered HWTX-I could alleviate neuropathic pain. [13] Cytosolic Ca2+ overload is one of the primary factors for inflammatory cells activation, therefore Ca2+ channel blockers can have a potential role as an anti-inflammatory drug. HWTX-I can relieve pain in the inflammatory joints and eliminate arthrocele to some degree. In a rat model of rheumatoid arthritis HWTX-I is able to decrease the concentration of tumor necrosis factor α (TNF-α) in serum and decrease the mRNA expression level interleukin 1β (IL-1β) and interleukin 6 (IL-6). [14]

Huwentoxin-X
HWTX-X is the smallest peptide among the huwentoxins so far isolated.
Chemistry
HWTX-X has a molecular mass of 2931 Da. It comprises 28 amino acid residues, including six cysteine residues forming three disulfide bridges. Like most huwentoxins, it adopts the ICK motif. [15] HWTX-X shows little homology with other huwentoxins, however, it can cause reversible blockage of N-type Ca2+ channels in rat dorsal root ganglion cells under whole-cell voltage clamp conditions. It does show more than 50% homology with the toxin Ptu1 from the assassin bug Peirates turpis and ω-conotoxin SVIA from the Conus striatus , two N-type Ca2+ blockers. [16]
Mode of action
HWTX-X has selectivity for isoforms of N-type Ca2+ channels, compared with ω-conotoxins GVIA and MVIIA.
Effects
HWTX-X specifically blocks GVIA-sensitive, N-type Ca2+ channels in rat dorsal root ganglion cells. It does not block L-type Ca2+ channels. While structurally similar to ω-conotoxins that block the twitch response to electrical nerve stimulation, HWTX-X has no effect on the twitch response of rat vas deferens. [15]