
Glutathione is an organic compound with the chemical formula HOCOCH(NH2)CH2CH2CONHCH(CH2SH)CONHCH2COOH. It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.

Aflatoxins are various poisonous carcinogens and mutagens that are produced by certain molds, particularly Aspergillus species mainly by Aspergillus flavus and Aspergillus parasiticus. According to the USDA, "They are probably the best known and most intensively researched mycotoxins in the world." The fungi grow in soil, decaying vegetation and various staple foodstuffs and commodities such as hay, maize, peanuts, coffee, wheat, millet, sorghum, cassava, rice, chili peppers, cottonseed, tree nuts, sesame seeds, sunflower seeds, and various cereal grains and oil seeds. In short, the relevant fungi grow on almost any crop or food. When such contaminated food is processed or consumed, the aflatoxins enter the general food supply. They have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Animals fed contaminated food can pass aflatoxin transformation products into milk, milk products, and meat. For example, contaminated poultry feed is the suspected source of aflatoxin-contaminated chicken meat and eggs in Pakistan.

Aspergillus niger is a mold classified within the Nigri section of the Aspergillus genus. The Aspergillus genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on decomposing matter, and suspended in the air. Species within this genus often grow quickly and can sporulate within a few days of germination. A combination of characteristics unique to A. niger makes the microbe invaluable to the production of many acids, proteins and bioactive compounds. Characteristics including extensive metabolic diversity, high production yield, secretion capability, and the ability to conduct post-translational modifications are responsible for A. niger's robust production of secondary metabolites. A. niger's capability to withstand extremely acidic conditions makes it especially important to the industrial production of citric acid.
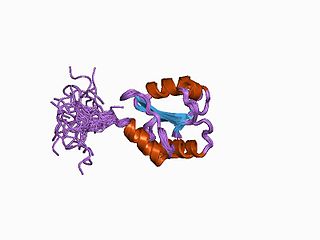
Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Aspergillus is a genus consisting of several hundred mold species found in various climates worldwide.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it can cause a variety of toxic effects, including kidney, liver and cell damage. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.

Sulfur assimilation is the process by which living organisms incorporate sulfur into their biological molecules. In plants, sulfate is absorbed by the roots and then be transported to the chloroplasts by the transipration stream where the sulfur are reduced to sulfide with the help of a series of enzymatic reactions. Furthermore, the reduced sulfur is incorporated into cysteine, an amino acid that is a precursor to many other sulfur-containing compounds. In animals, sulfur assimilation occurs primarily through the diet, as animals cannot produce sulfur-containing compounds directly. Sulfur is incorporated into amino acids such as cysteine and methionine, which are used to build proteins and other important molecules.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
Adenylyl-sulfate reductase (thioredoxin) is an enzyme that catalyzes the chemical reaction
Pathogenic fungi are fungi that cause disease in humans or other organisms. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. Approximately 300 fungi are known to be pathogenic to humans; their study is called "medical mycology". Fungal infections are estimated to kill more people than either tuberculosis or malaria—about two million people per year.

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.

Aflatoxin B1 is an aflatoxin produced by Aspergillus flavus and A. parasiticus. It is a very potent carcinogen with a TD50 3.2 μg/kg/day in rats. This carcinogenic potency varies across species with some, such as rats and monkeys, seemingly much more susceptible than others. Aflatoxin B1 is a common contaminant in a variety of foods including peanuts, cottonseed meal, corn, and other grains; as well as animal feeds. Aflatoxin B1 is considered the most toxic aflatoxin and it is highly implicated in hepatocellular carcinoma (HCC) in humans. In animals, aflatoxin B1 has also been shown to be mutagenic, teratogenic, and to cause immunosuppression. Several sampling and analytical methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry, and enzyme-linked immunosorbent assay (ELISA), among others, have been used to test for aflatoxin B1 contamination in foods. According to the Food and Agriculture Organization (FAO), a division of the United Nations, the worldwide maximum tolerated levels of aflatoxin B1 was reported to be in the range of 1–20 μg/kg (or .001 ppm - 1 part-per-billion) in food, and 5–50 μg/kg (.005 ppm) in dietary cattle feed in 2003.

Bacterial glutathione transferases are part of a superfamily of enzymes that play a crucial role in cellular detoxification. The primary role of GSTs is to catalyze the conjugation of glutathione (GSH) with the electrophilic centers of a wide variety of molecules. The most commonly known substrates of GSTs are xenobiotic synthetic chemicals. There are also classes of GSTs that utilize glutathione as a cofactor rather than a substrate. Often these GSTs are involved in reduction of reactive oxidative species toxic to the bacterium. Conjugation with glutathione receptors renders toxic substances more soluble, and therefore more readily exocytosed from the cell.

Chronic pulmonary aspergillosis is a long-term fungal infection caused by members of the genus Aspergillus—most commonly Aspergillusfumigatus. The term describes several disease presentations with considerable overlap, ranging from an aspergilloma—a clump of Aspergillus mold in the lungs—through to a subacute, invasive form known as chronic necrotizing pulmonary aspergillosis which affects people whose immune system is weakened. Many people affected by chronic pulmonary aspergillosis have an underlying lung disease, most commonly tuberculosis, allergic bronchopulmonary aspergillosis, asthma, or lung cancer.
Chanoclavine-I dehydrogenase (EC 1.1.1.332, easD (gene), fgaDH (gene)) is an enzyme with systematic name chanoclavine-I:NAD+ oxidoreductase. This enzyme catalises the following chemical reaction

Pseurotin A is a secondary metabolite of Aspergillus.

L-ornithine N5 monooxygenase (EC 1.14.13.195 or EC 1.14.13.196) is an enzyme which catalyzes one of the following chemical reactions:
L-ornithine + NADPH + O2 N(5)-hydroxy-L-ornithine + NADP+ + H2O L-ornithine + NAD(P)H + O2 N(5)-hydroxy-L-ornithine + NAD(P)+ + H2O
Aspergillus viridinutans is a species of fungus in the genus Aspergillus. The species was first isolated in Frankston, Victoria, Australia and described in 1954. It is from the Fumigati section of Aspergillus. Several fungi from this section produce heat-resistant ascospores, and the isolates from this section are frequently obtained from locations where natural fires have previously occurred. A. viridinutans has been identified as the cause of chronic aspergillosis. The mycotoxin viriditoxin was first identified in A. viridinutans. A draft genome sequence of the strain derived from the original species description has been generated.
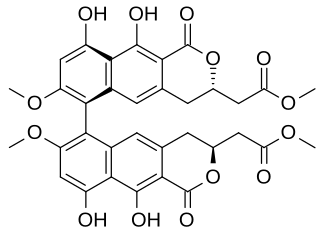
Viriditoxin (VDT) is a secondary metabolite produced by fungi. Viriditoxin is a type of mycotoxin. The biosynthesis of the compound has been investigated.

















