Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

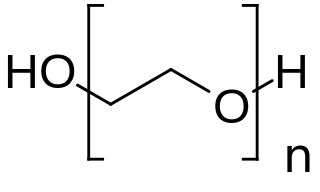
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

Post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes translating mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.
Sephadex is a cross-linked dextran gel used for gel filtration. It was launched by Pharmacia in 1959, after development work by Jerker Porath and Per Flodin. The name is derived from separation Pharmacia dextran. It is normally manufactured in a bead form and most commonly used for gel filtration columns. By varying the degree of cross-linking, the fractionation properties of the gel can be altered.
Mixed-mode chromatography (MMC), or multimodal chromatography, refers to chromatographic methods that utilize more than one form of interaction between the stationary phase and analytes in order to achieve their separation. What is distinct from conventional single-mode chromatography is that the secondary interactions in MMC cannot be too weak, and thus they also contribute to the retention of the solutes.
Pegylated interferon (PEG-IFN) is a class of medication that includes three different drugs as of 2012:
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks. It is also used as an excipient. It is also used to clear the bowels before a colonoscopy, when the onset of the laxative effect is more rapid, typically within an hour.
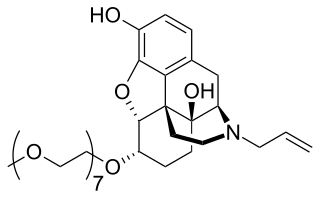
Naloxegol is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.
Thermoresponsive polymers can be used as stationary phase in liquid chromatography. Here, the polarity of the stationary phase can be varied by temperature changes, altering the power of separation without changing the column or solvent composition. Thermally related benefits of gas chromatography can now be applied to classes of compounds that are restricted to liquid chromatography due to their thermolability. In place of solvent gradient elution, thermoresponsive polymers allow the use of temperature gradients under purely aqueous isocratic conditions. The versatility of the system is controlled not only through changing temperature, but through the addition of modifying moieties that allow for a choice of enhanced hydrophobic interaction, or by introducing the prospect of electrostatic interaction. These developments have already introduced major improvements to the fields of hydrophobic interaction chromatography, size exclusion chromatography, ion exchange chromatography, and affinity chromatography separations as well as pseudo-solid phase extractions.

A nanocarrier is nanomaterial being used as a transport module for another substance, such as a drug. Commonly used nanocarriers include micelles, polymers, carbon-based materials, liposomes and other substances. Nanocarriers are currently being studied for their use in drug delivery and their unique characteristics demonstrate potential use in chemotherapy. This class of materials was first reported by a team of researchers of University of Évora, Alentejo in early 1960's, and grew exponentially in relevance since then.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
Hydrogels are three-dimensional networks consisting of chemically or physically cross-linked hydrophilic polymers. The insoluble hydrophilic structures absorb polar wound exudates and allow oxygen diffusion at the wound bed to accelerate healing. Hydrogel dressings can be designed to prevent bacterial infection, retain moisture, promote optimum adhesion to tissues, and satisfy the basic requirements of biocompatibility. Hydrogel dressings can also be designed to respond to changes in the microenvironment at the wound bed. Hydrogel dressings should promote an appropriate microenvironment for angiogenesis, recruitment of fibroblasts, and cellular proliferation.

Polymer-protein hybrids are a class of nanostructure composed of protein-polymer conjugates. The protein component generally gives the advantages of biocompatibility and biodegradability, as many proteins are produced naturally by the body and are therefore well tolerated and metabolized. Although proteins are used as targeted therapy drugs, the main limitations—the lack of stability and insufficient circulation times still remain. Therefore, protein-polymer conjugates have been investigated to further enhance pharmacologic behavior and stability. By adjusting the chemical structure of the protein-polymer conjugates, polymer-protein particles with unique structures and functions, such as stimulus responsiveness, enrichment in specific tissue types, and enzyme activity, can be synthesized. Polymer-protein particles have been the focus of much research recently because they possess potential uses including bioseparations, imaging, biosensing, gene and drug delivery.
Peptide therapeutics are peptides or polypeptides which are used to for the treatment of diseases. Naturally occurring peptides may serve as hormones, growth factors, neurotransmitters, ion channel ligands, and anti-infectives; peptide therapeutics mimic such functions. Peptide Therapeutics are seen as relatively safe and well-tolerated as peptides can be metabolized by the body.
Glycopegylation "is a site-selective PEGylation method developed for modifying complex glycoproteins". It can be useful to improve bioavailability and extend the half-life of various therapeutic proteins. Examples of glycopegylated molecules include pegozafermin and recombinant factor IX.
References
- 1 2 Fee, Conan (18 September 2009). PEGylated protein drugs : basic science and clinical applications. Birkhäuser. pp. 113–124. ISBN 978-3-7643-8678-8.
- 1 2 3 4 5 6 7 8 da Silva Freitas, Débora; Abrahão-Neto, José (15 June 2010). "Biochemical and biophysical characterization of lysozyme modified by PEGylation". International Journal of Pharmaceutics. 392 (1–2): 111–117. doi: 10.1016/j.ijpharm.2010.03.036 . PMID 20307635.
- ↑ "Characterisation Studies of PEGylated Lysozyme". The Analytical Scientist. 29 September 2014. Retrieved 2020-07-17.
- 1 2 3 4 5 6 7 da Silva Freitas, Débora; Abrahão-Neto, José (2010-06-15). "Biochemical and biophysical characterization of lysozyme modified by PEGylation". International Journal of Pharmaceutics. 392 (1–2): 111–117. doi: 10.1016/j.ijpharm.2010.03.036 . PMID 20307635.
- 1 2 3 4 5 6 7 Pai, Sheetal S.; Hammouda, Boualem; Hong, Kunlun; Pozzo, Danilo C.; Przybycien, Todd M.; Tilton, Robert D. (2011-11-16). "The Conformation of the Poly(ethylene glycol) Chain in Mono-PEGylated Lysozyme and Mono-PEGylated Human Growth Hormone". Bioconjugate Chemistry. 22 (11): 2317–2323. doi:10.1021/bc2003583. ISSN 1043-1802. PMID 21950579.
- 1 2 Morgenstern, Josefine; Baumann, Pascal; Brunner, Carina; Hubbuch, Jürgen (2017-01-19). "Effect of PEG molecular weight and PEGylation degree on the physical stability of PEGylated lysozyme". International Journal of Pharmaceutics. 519 (1–2): 408–417. doi:10.1016/j.ijpharm.2017.01.040. PMID 28130198.
- 1 2 3 4 5 Maiser, Benjamin; Dismer, Florian; Hubbuch, Jürgen (2014). "Optimization of random PEGylation reactions by means of high throughput screening". Biotechnology and Bioengineering. 111 (1): 104–114. doi:10.1002/bit.25000. ISSN 1097-0290. PMID 23939788. S2CID 205503389.
- ↑ Ottow, Kim Ekelund; Lund‐Olesen, Torsten; Maury, Trine Lütken; Hansen, Mikkel Fougt; Hobley, Timothy J. (2011). "A magnetic adsorbent-based process for semi-continuous PEGylation of proteins". Biotechnology Journal. 6 (4): 396–409. doi:10.1002/biot.201000360. ISSN 1860-7314. PMID 21259443.
- ↑ Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. (2014-12-11). "Sequential separation of lysozyme, ovomucin, ovotransferrin, and ovalbumin from egg white". Poultry Science. 93 (4): 1001–1009. doi: 10.3382/ps.2013-03403 . PMID 24706978.
- ↑ Fee, Conan J.; Van Alstine, James M. (2005-11-08). "PEG-proteins: Reaction engineering and separation issues". Chemical Engineering Science. 61 (3): 924–939. doi:10.1016/j.ces.2005.04.040.
- ↑ Lee, K. C.; Tak, K. K.; Park, M. O.; Lee, J. T.; Woo, B. H.; Yoo, S. D.; Lee, H. S.; DeLuca, P. P. (1999-05-01). "Preparation and characterization of polyethylene-glycol-modified salmon calcitonins". Pharmaceutical Development and Technology. 4 (2): 269–275. doi:10.1081/pdt-100101361. ISSN 1083-7450. PMID 10231888.
- 1 2 Imoto, Taiji; Yagishita, Kazuyoshi (1971-04-24). "A Simple Activity Measurement of Lysozyme". Agricultural and Biological Chemistry. 35 (7): 1154–1156. doi:10.1080/00021369.1971.10860050. ISSN 0002-1369.
- 1 2 Shugar, David (1952-03-29). "The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme". Biochimica et Biophysica Acta. 8 (3): 302–309. doi:10.1016/0006-3002(52)90045-0. PMID 14934741.
- ↑ Nodake, Yuichi; Yamasaki, Nobuyuki (1999-11-25). "Some Properties of a Macromolecular Conjugate of Lysozyme Prepared by Modification with a Monomethoxypolyethylene Glycol Derivative". Bioscience, Biotechnology, and Biochemistry. 64 (4): 767–774. doi: 10.1271/bbb.64.767 . ISSN 0916-8451. PMID 10830491. S2CID 2851002.