Related Research Articles
An intermolecular force (IMF) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
A plasma afterglow is the radiation emitted from a plasma after the source of ionization is removed. The external electromagnetic fields that sustained the plasma glow are absent or insufficient to maintain the discharge in the afterglow. A plasma afterglow can either be a temporal, due to an interrupted (pulsed) plasma source, or spatial, due to a distant plasma source. In the afterglow, plasma-generated species de-excite and participate in secondary chemical reactions that tend to form stable species. Depending on the gas composition, super-elastic collisions may continue to sustain the plasma in the afterglow for a while by releasing the energy stored in rovibronic degrees of freedom of the atoms and molecules of the plasma. Especially in molecular gases, the plasma chemistry in the afterglow is significantly different from the plasma glow. The afterglow of a plasma is still a plasma and as thus retains most of the properties of a plasma.

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation.

Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber at the University of Manchester in 1980. When a beam of high energy ions is used instead of atoms, the method is known as liquid secondary ion mass spectrometry (LSIMS). In FAB and LSIMS, the material to be analyzed is mixed with a non-volatile chemical protection environment, called a matrix, and is bombarded under vacuum with a high energy atomic beam. The atoms are typically from an inert gas such as argon or xenon. Common matrices include glycerol, thioglycerol, 3-nitrobenzyl alcohol (3-NBA), 18-crown-6 ether, 2-nitrophenyloctyl ether, sulfolane, diethanolamine, and triethanolamine. This technique is similar to secondary ion mass spectrometry and plasma desorption mass spectrometry.
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important applications for this science is in studying the thermodynamics and kinetics of reactions. For example, one application is in studying the thermodynamics of the solvation of ions. Ions with small solvation spheres of 1, 2, 3... solvent molecules can be studied in the gas phase and then extrapolated to bulk solution.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.

The helium hydride ion, hydridohelium(1+) ion, or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.

Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules. In the collision, some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry.
Xenon monochloride (XeCl) is an exciplex which is used in excimer lasers and excimer lamps emitting near ultraviolet light at 308 nm. It is most commonly used in medicine. Xenon monochloride was first synthesized in the 1960s. Its kinetic scheme is very complex and its state changes occur on a nanosecond timescale. In the gaseous state, at least two kinds of xenon monochloride are known: XeCl and Xe
2Cl, whereas complex aggregates form in the solid state in noble gas matrices. The excited state of xenon resembles halogens and it reacts with them to form excited molecular compounds.

Mercury(II) hydride is an inorganic compound with the chemical formula HgH
2. It is both thermodynamically and kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it known as a white, crystalline solid, which is kinetically stable at temperatures below −125 °C (−193 °F), which was synthesised for the first time in 1951.
An excimer lamp is a source of ultraviolet light based on spontaneous emission of excimer (exciplex) molecules.

Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
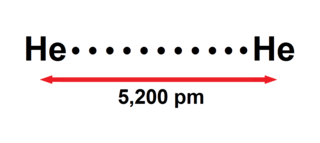
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.

Infrared photodissociation (IRPD) spectroscopy uses infrared radiation to break bonds in, often ionic, molecules (photodissociation), within a mass spectrometer. In combination with post-ionization, this technique can also be used for neutral species. IRPD spectroscopy has been shown to use electron ionization, corona discharge, and electrospray ionization to obtain spectra of volatile and nonvolatile compounds. Ionized gases trapped in a mass spectrometer can be studied without the need of a solvent as in infrared spectroscopy.

Diargon or the argon dimer is a molecule containing two argon atoms. Normally, this is only very weakly bound together by van der Waals forces. However, in an excited state, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperatures, argon gas can have a few percent of diargon molecules.
References
- 1 2 3 4 5 Pilgrim, J. S.; Yeh, C. S.; Berry, K. R.; Duncan, M. A. (1994). "Photodissociation spectroscopy of Mg+–rare gas complexes". The Journal of Chemical Physics. 100 (11): 7945. Bibcode:1994JChPh.100.7945P. doi:10.1063/1.466840.
- 1 2 Bauschlicher, Charles W.; Partridge, Harry (June 1995). "A study of the X 2Σ+ and A 2Π states of MgAr+ and MgKr+". Chemical Physics Letters. 239 (4–6): 241–245. Bibcode:1995CPL...239..241B. doi:10.1016/0009-2614(95)00449-E.
- ↑ Massick, Steven; Breckenridge, W.H. (August 1996). "A determination of the ionization threshold for the Mg(3s3p3P0) · Ar(3Π0−) metastable state: The bond energy of MgAr+". Chemical Physics Letters. 257 (5–6): 465–470. Bibcode:1996CPL...257..465M. doi:10.1016/0009-2614(96)00565-9.
- ↑ Massick, Steven; Breckenridge, W. H. (8 February 1997). "Spectroscopic characterization of the 3Δ(4d), 3Π(4d), 3Σ+(4d), and 3Π(5p) Rydberg states of the MgAr van der Waals molecule". The Journal of Chemical Physics. 106 (6): 2171–2181. Bibcode:1997JChPh.106.2171M. doi:10.1063/1.473673.
- 1 2 3 4 Leung, Allen W.K.; Roberson, Mark; Simons, Jack; Breckenridge, W.H. (August 1996). "Strong bonding in a doubly excited valence state of a van der Waals molecule". Chemical Physics Letters. 259 (1–2): 199–203. Bibcode:1996CPL...259..199L. doi:10.1016/0009-2614(96)00723-3.
- 1 2 3 4 5 6 Massick, Steven; Breckenridge, W. H. (8 December 1996). "Spectroscopic characterization of the excited Mg(3s3d 3DJ)⋅Ar(3Π), Mg(3s3d 2DJ)⋅Ar(3Δ), and Mg(3s4p 3PJ)⋅Ar(3Π) van der Waals states". The Journal of Chemical Physics. 105 (22): 9719–9732. Bibcode:1996JChPh.105.9719M. doi:10.1063/1.472843.
- 1 2 3 Hald, Kasper; Jørgensen, Poul; Breckenridge, W.H; Jaszuński, Michał (October 2002). "Calculation of ground and excited state potential energy curves of the MgAr complex using the coupled cluster approximate triples model CC3". Chemical Physics Letters. 364 (3–4): 402–408. Bibcode:2002CPL...364..402H. doi:10.1016/S0009-2614(02)01339-8.
- ↑ Massick, Steven; Breckenridge, W. H. (15 May 1996). "A new class of strongly bound, doubly excited valence states of neutral van der Waals molecules: Mg(3pπ,3pπ 3PJ )⋅Ar(3Σ)". The Journal of Chemical Physics. 104 (19): 7784–7787. Bibcode:1996JChPh.104.7784M. doi:10.1063/1.471657.
- 1 2 3 4 5 Hüttner, W. (2012). "10 AlH X 1Σ+ Aluminum hydride". Molecular Constants Mostly from Microwave, Molecular Beam, and Sub-Doppler Laser Spectroscopy: Diamagnetic Diatomic Molecules. Part 1. Landolt-Börnstein - Group II Molecules and Radicals. Vol. 29A1. Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology. Springer. p. 53. Bibcode:2012LanB.29A1...25H. doi:10.1007/978-3-540-69954-5_12. ISBN 978-3-540-69953-8. ISSN 1615-1852.
- ↑ Miao, Mao-sheng; Wang, Xiao-li; Brgoch, Jakoah; Spera, Frank; Jackson, Matthew G.; Kresse, Georg; Lin, Hai-qing (11 November 2015). "Anionic Chemistry of Noble Gases: Formation of Mg?NG (NG = Xe, Kr, Ar) Compounds under Pressure". Journal of the American Chemical Society. 137 (44): 14122–14128. doi:10.1021/jacs.5b08162. PMID 26488848.
- ↑ Mason, Thomas F. D.; Weiss, Dominik J.; Horstwood, Matthew; Parrish, Randall R.; Russell, Sara S.; Mullane, Eta; Coles, Barry J. (2004). "High-precision Cu and Zn isotope analysis by plasma source mass spectrometry". Journal of Analytical Atomic Spectrometry. 19 (2): 209. doi:10.1039/b306958c.
- ↑ Duckworth, Douglas C.; Barshick, Christopher M.; Smith, David H. (1993). "Analysis of soils by glow discharge mass spectrometry". Journal of Analytical Atomic Spectrometry. 8 (6): 875. doi:10.1039/JA9930800875.