
Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate.

Hydrogen sulfide is a chemical compound with the formula H2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures is stinkdamp. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777.

Methanethiol is an organosulfur compound with the chemical formula CH
3SH. It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals, as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable.

Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula PH3, classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (P2H4). With traces of P2H4 present, PH3 is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure.

Sterilization refers to any process that removes, kills, or deactivates all forms of life and other biological agents present in or on a specific surface, object, or fluid. Sterilization can be achieved through various means, including heat, chemicals, irradiation, high pressure, and filtration. Sterilization is distinct from disinfection, sanitization, and pasteurization, in that those methods reduce rather than eliminate all forms of life and biological agents present. After sterilization, an object is referred to as being sterile or aseptic.

Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.

MAPP gas was a trademarked name, belonging to The Linde Group, a division of the former global chemical giant Union Carbide, for a fuel gas based on a stabilized mixture of methylacetylene (propyne), propadiene and propane. The name comes from the original chemical composition, methylacetylene-propadiene propane. "MAPP gas" is also widely used as a generic name for UN 1060 stabilised methylacetylene-propadiene.

The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment." Examples include smoke or other poisonous gases at sufficiently high concentrations. It is calculated using the LD50 or LC50. The Occupational Safety and Health Administration (OSHA) regulation defines the term as "an atmosphere that poses an immediate threat to life, would cause irreversible adverse health effects, or would impair an individual's ability to escape from a dangerous atmosphere."
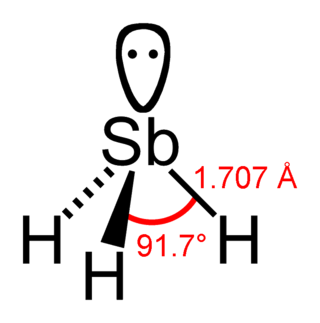
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs).

Sewer gas is a complex, generally obnoxious smelling mixture of toxic and nontoxic gases produced and collected in sewage systems by the decomposition of organic household or industrial wastes, typical components of sewage.

Ethyl formate is an ester formed when ethanol reacts with formic acid. Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries. It occurs naturally in the body of ants and in the stingers of bees.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.
An anaerobic lagoon or manure lagoon is a man-made outdoor earthen basin filled with animal waste that undergoes anaerobic respiration as part of a system designed to manage and treat refuse created by concentrated animal feeding operations (CAFOs). Anaerobic lagoons are created from a manure slurry, which is washed out from underneath the animal pens and then piped into the lagoon. Sometimes the slurry is placed in an intermediate holding tank under or next to the barns before it is deposited in a lagoon. Once in the lagoon, the manure settles into two layers: a solid or sludge layer and a liquid layer. The manure then undergoes the process of anaerobic respiration, whereby the volatile organic compounds are converted into carbon dioxide and methane. Anaerobic lagoons are usually used to pretreat high strength industrial wastewaters and municipal wastewaters. This allows for preliminary sedimentation of suspended solids as a pretreatment process.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.
Sterilant gas monitoring is the detection of hazardous gases used by health care and other facilities to sterilize medical supplies that cannot be sterilized by heat or steam methods. The current FDA approved sterilant gases are ethylene oxide, hydrogen peroxide and ozone. Other liquid sterilants, such as peracetic acid, may also be used for sterilization and may raise similar occupational health issues. Sterilization means the complete destruction of all biological life, and sterilization efficacy is typically considered adequate if less than one in a million microbes remain viable.
Vaporized hydrogen peroxide (trademarked VHP, also known as hydrogen peroxide vapor, HPV) is a vapor form of hydrogen peroxide (H2O2) with applications as a low-temperature antimicrobial vapor used to decontaminate enclosed and sealed areas such as laboratory workstations, isolation and pass-through rooms, and even aircraft interiors.
Hydrogen sulfide (H2S) is a noxious gas characterized by its distinctive stench reminiscent of rotten eggs. It goes by several colloquial names, including sewer gas, stink damp, swamp gas, and manure gas. This gas naturally occurs in crude petroleum, natural gas, hot springs, and certain food items. In the natural world, H2S is a common byproduct of the decomposition of organic matter, such as human and animal waste, in septic and sewer systems due to bacterial processes. Additionally, it is industrially produced in significant quantities through activities and facilities like petroleum and natural gas extraction, refining, wastewater treatment, coke ovens, tanneries, kraft paper mills, and landfills.

Sulfotep (also known as tetraethyldithiopyrophosphate and TEDP) is a pesticide commonly used in greenhouses as a fumigant. The substance is also known as Dithione, Dithiophos, and many other names. Sulfotep has the molecular formula C8H20O5P2S2 and belongs to the organophosphate class of chemicals. It has a cholinergic effect, involving depression of the cholinesterase activity of the peripheral and central nervous system of insects. The transduction of signals is disturbed at the synapses that make use of acetylcholine. Sulfotep is a mobile oil that is pale yellow-colored and smells like garlic. It is primarily used as an insecticide.
Hydrogen sulfide is produced in small amounts by some cells of the mammalian body and has a number of biological signaling functions. Only two other such gases are currently known: nitric oxide (NO) and carbon monoxide (CO).














