
Pesticide resistance describes the decreased susceptibility of a pest population to a pesticide that was previously effective at controlling the pest. Pest species evolve pesticide resistance via natural selection: the most resistant specimens survive and pass on their acquired heritable changes traits to their offspring. If a pest has resistance then that will reduce the pesticide's efficacy – efficacy and resistance are inversely related.
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, although sharing similar methods of infecting plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward. Most fungicides that can be bought retail are sold in liquid form, the active ingredient being present at 0.08% in weaker concentrates, and as high as 0.5% for more potent fungicides. Fungicides in powdered form are usually around 90% sulfur.

An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally.

Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential.
A biopesticide is a biological substance or organism that damages, kills, or repels organisms seen as pests. Biological pest management intervention involves predatory, parasitic, or chemical relationships.
The cereal grain wheat is subject to numerous wheat diseases, including bacterial, viral and fungal diseases, as well as parasitic infestations.

Acibenzolar-S-methyl is the ISO common name for an organic compound that is used as a fungicide. Unusually, it is not directly toxic to fungi but works by inducing systemic acquired resistance, the natural defence system of plants.

Alternaria solani is a fungal pathogen that produces a disease in tomato and potato plants called early blight. The pathogen produces distinctive "bullseye" patterned leaf spots and can also cause stem lesions and fruit rot on tomato and tuber blight on potato. Despite the name "early," foliar symptoms usually occur on older leaves. If uncontrolled, early blight can cause significant yield reductions. Primary methods of controlling this disease include preventing long periods of wetness on leaf surfaces and applying fungicides. Early blight can also be caused by Alternaria tomatophila, which is more virulent on stems and leaves of tomato plants than Alternaria solani.

Helminthosporium solani is a fungal plant pathogen responsible for the plant disease known as silver scurf. Silver scurf is a blemish disease, meaning the effect it has on tubers is mostly cosmetic and affects "fresh market, processing and seed tuber potatoes." There are some reports of it affecting development, meaning growth and tuber yield. This is caused by light brown lesions, which in turn change the permeability of tuber skin and then it causes tuber shrinkage and water loss, which finally causes weight loss. The disease has become economically important because silver scurf affected potatoes for processing and direct consumption have been rejected by the industry. The disease cycle can be divided into two stages: field and storage. It is mainly a seed borne disease and the primary source of inoculum is mainly infected potato seed tubers. Symptoms develop and worsen in storage because the conditions are conducive to sporulation. The ideal conditions for the spread of this disease are high temperatures and high humidity. There are also many cultural practices that favor spread and development. There are multiple ways to help control the disease.

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.
Copper pesticides are copper compounds used as bactericides, algaecides, or fungicides. They can kill bacteria, oomycetes and algae, and prevent fungal spores from germinating. Common forms of fixed copper fungicides include copper sulfate, copper sulfate pentahydrate, copper hydroxide, copper oxychloride sulfate, cuprous oxide, and copper octanoate.

Fluxapyroxad is a broad-spectrum pyrazole-carboxamide fungicide used on a large variety of commercial crops. It stunts fungus growth by inhibiting the succinate dehydrogenase (SQR) enzyme. Application of fluxapyroxad helps prevent many wilts and other fungal infections from taking hold. As with other systemic pesticides that have a long chemical half-life, there are concerns about keeping fluxapyroxad out of the groundwater, especially when combined with pyraclostrobin. There is also concern that some fungi may develop resistance to fluxapyroxad.

Cyproconazole is an agricultural fungicide of the class of azoles, used on cereal crops, coffee, sugar beet, fruit trees and grapes, on sod farms and golf courses and on wood as a preservative. It was introduced to the market by then Sandoz in 1994.
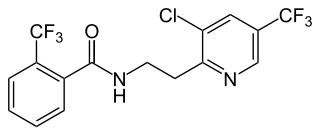
Fluopyram is a fungicide and nematicide used in agriculture. It is used to control fungal diseases such as gray mold, powdery mildew, apple scab, Alternaria, Sclerotinia, and Monilinia. It is an inhibitor of succinate dehydrogenase.

Oxycarboxin is an organic chemical used in agriculture to protect crops from fungal diseases. It was first marketed by Uniroyal in 1969 using their brand name Plantvax. The compound is an anilide which combines a heterocyclic acid with aniline to give an inhibitor of succinate dehydrogenase (SDHI).
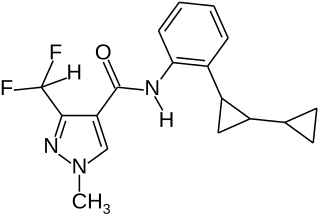
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase.
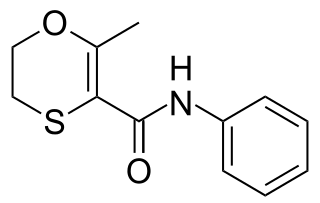
Carboxin is a narrow-spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Uniroyal in 1969 using their brand name Vitavax. The compound is an anilide which combines a heterocyclic acid with aniline to give an inhibitor of succinate dehydrogenase (SDHI).

Boscalid is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by BASF in 2002 using their brand name Endura. The compound is an biphenyl amide derived inhibitor of succinate dehydrogenase.

Pydiflumetofen is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an amide which combines a pyrazole acid with a substituted phenethylamine to give an inhibitor of succinate dehydrogenase, an enzyme that inhibits cellular respiration in almost all living organisms.

3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is a chemical compound which is used commercially as an intermediate to seven fungicides which act by inhibition of succinate dehydrogenase (SDHI). It consists of a pyrazole ring with difluoromethyl, methyl and carboxylic acid groups attached in specific positions.
















