
Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.
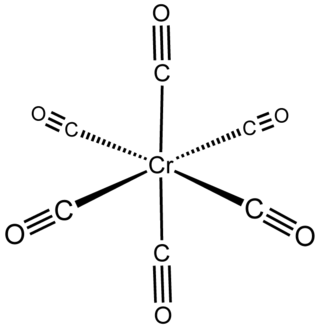
Chromium hexacarbonyl (IUPAC name: hexacarbonylchromium) is a chromium(0) organometallic compound with the formula Cr(CO)6. It is homoleptic complex, which means that all the ligands are identical. It is a white, air-stable solid with a high vapor pressure.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.

In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.

Triiron dodecarbonyl is the organoiron compound with the formula Fe3(CO)12. It is a dark green solid that sublimes under vacuum. It is soluble in nonpolar organic solvents to give intensely green solutions. Most low-nuclearity clusters are pale yellow or orange. Hot solutions of Fe3(CO)12 decompose to an iron mirror, which can be pyrophoric in air.The solid decomposes slowly in air, and thus samples are typically stored cold under an inert atmosphere. It is a more reactive source of iron(0) than iron pentacarbonyl.
Walter Hieber was an inorganic chemist, known as the father of metal carbonyl chemistry. He was born 18 December 1895 and died 29 November 1976. Hieber's father was Johannes Hieber, an influential evangelical minister and politician.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.
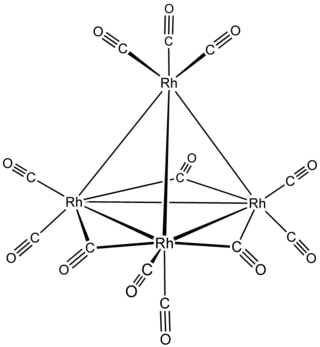
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic synthesis.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Hexadecacarbonylhexarhodium is a metal carbonyl cluster with the formula Rh6(CO)16. It exists as purple-brown crystals that are slightly soluble in dichloromethane and chloroform. It is the principal binary carbonyl of rhodium.
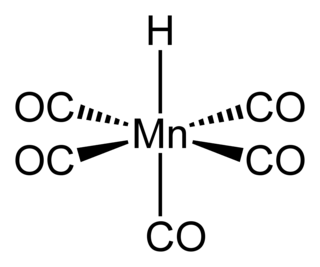
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.

Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Metal carbonyl hydrides are complexes of transition metals with carbon monoxide and hydride as ligands. These complexes are useful in organic synthesis as catalysts in homogeneous catalysis, such as hydroformylation.

Carbonyl hydrido tris(triphenylphosphine)rhodium(I) [Carbonyl(hydrido)tris(triphenylphosphane)rhodium(I)] is an organorhodium compound with the formula [RhH(CO)(PPh3)3] (Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation.

Rhodium carbonyl chloride is an organorhodium compound with the formula Rh2Cl2(CO)4. It is a red-brown volatile solid that is soluble in nonpolar organic solvents. It is a precursor to other rhodium carbonyl complexes, some of which are useful in homogeneous catalysis.

In the area of metal cluster chemistry, a butterfly cluster compound usually describes tetrametallic clusters containing five M-M bonds. A prototype of this motif is [Re4(CO)16]2−. Most butterfly clusters have additional bridging ligands. One example is the pentaphosphide [[Rh4(CO)5(PPh2)5]]− where all Rh---Rh edges are bridged by PPh2. A carbide-containing butterfly cluster is [Fe4C(CO)12]2− where the carbide is bonded to all four Fe centers.

Tetrabutylammonium is a quaternary ammonium cation with the formula [N(C4H9)4]+, also denoted [NBu4]+. It is used in the research laboratory to prepare lipophilic salts of inorganic anions. Relative to tetraethylammonium derivatives, tetrabutylammonium salts are more lipophilic but crystallize less readily.

Paolo Chini (1928–1980) was an Italian chemist, known as the "King of the Clusters". He was a pioneer in metal carbonyl cluster syntheses.

Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions.


![The carbido cluster [Os10C(CO)24] . The bent OsCO units are an artifact of the crystallographic analysis. Os10(CO)24 2-.png](http://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Os10%28CO%2924_2-.png/260px-Os10%28CO%2924_2-.png)
















