
Geophysics is a subject of natural science concerned with the physical processes and physical properties of the Earth and its surrounding space environment, and the use of quantitative methods for their analysis. Geophysicists, who usually study geophysics, physics, or one of the Earth sciences at the graduate level, complete investigations across a wide range of scientific disciplines. The term geophysics classically refers to solid earth applications: Earth's shape; its gravitational, magnetic fields, and electromagnetic fields ; its internal structure and composition; its dynamics and their surface expression in plate tectonics, the generation of magmas, volcanism and rock formation. However, modern geophysics organizations and pure scientists use a broader definition that includes the water cycle including snow and ice; fluid dynamics of the oceans and the atmosphere; electricity and magnetism in the ionosphere and magnetosphere and solar-terrestrial physics; and analogous problems associated with the Moon and other planets.
In science and engineering the study of high pressure examines its effects on materials and the design and construction of devices, such as a diamond anvil cell, which can create high pressure. By high pressure is usually meant pressures of thousands (kilobars) or millions (megabars) of times atmospheric pressure.
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor. This phase was predicted in 1935 on theoretical grounds by Eugene Wigner and Hillard Bell Huntington.
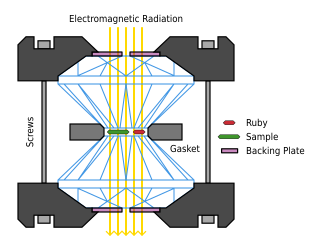
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It enables the compression of a small (sub-millimeter-sized) piece of material to extreme pressures, typically up to around 100–200 gigapascals, although it is possible to achieve pressures up to 770 gigapascals.
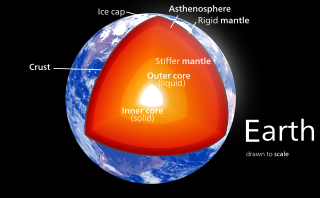
The internal structure of Earth is the layers of the Earth, excluding its atmosphere and hydrosphere. The structure consists of an outer silicate solid crust, a highly viscous asthenosphere and solid mantle, a liquid outer core whose flow generates the Earth's magnetic field, and a solid inner core.
Post-perovskite (pPv) is a high-pressure phase of magnesium silicate (MgSiO3). It is composed of the prime oxide constituents of the Earth's rocky mantle (MgO and SiO2), and its pressure and temperature for stability imply that it is likely to occur in portions of the lowermost few hundred km of Earth's mantle.

Omphacite is a member of the clinopyroxene group of silicate minerals with formula: (Ca, Na)(Mg, Fe2+, Al)Si2O6. It is a variably deep to pale green or nearly colorless variety of clinopyroxene. It normally appears in eclogite, which is the high-pressure metamorphic rock of basalt. Omphacite is the solid solution of Fe-bearing diopside and jadeite. It crystallizes in the monoclinic system with prismatic, typically twinned forms, though usually anhedral. Its space group can be P2/n or C2/c depending on the thermal history. It exhibits the typical near 90° pyroxene cleavage. It is brittle with specific gravity of 3.29 to 3.39 and a Mohs hardness of 5 to 6.
A multi-anvil press, or anvil press is a type of device related to a machine press that is used to create extraordinarily high pressures within a small volume.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).

Ho-Kwang (Dave) Mao is a Chinese-American geologist. He is the director of the Center for High Pressure Science and Technology Advanced Research in Shanghai, China. He was a staff scientist at Geophysical Laboratory of the Carnegie Institution for Science for more than 30 years. Mao is a recognized leading scientist in high pressure geosciences and physical science. There are two minerals named after him, Davemaoite and Maohokite.

Albert Francis Birch was an American geophysicist. He is considered one of the founders of solid Earth geophysics. He is also known for his part in the atomic bombing of Hiroshima and Nagasaki.

Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above 120 K (−153 °C). Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least 715 K (442 °C) and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt.
Pyrolite is a term used to characterize a model composition of the Earth's mantle. This model is based on that a pyrolite source can produce the Mid-Ocean Ridge Basalt by partial melting. It was first proposed by Ted Ringwood (1962) as being 1 part basalt and 4 parts harzburgite, but later was revised to being 1 part tholeiitic basalt and 3 parts dunite. The term is derived from the mineral names PYR-oxene and OL-ivine. However, whether pyrolite is representative of the Earth's mantle remains debated.
Birch's law, discovered by the geophysicist Francis Birch, establishes a linear relation between compressional wave velocity vp and density of rocks and minerals:
The Adams–Williamson equation, named after Leason H. Adams and E. D. Williamson, is an equation used to determine density as a function of radius, more commonly used to determine the relation between the velocities of seismic waves and the density of the Earth's interior. Given the average density of rocks at the Earth's surface and profiles of the P-wave and S-wave speeds as function of depth, it can predict how density increases with depth. It assumes that the compression is adiabatic and that the Earth is spherically symmetric, homogeneous, and in hydrostatic equilibrium. It can also be applied to spherical shells with that property. It is an important part of models of the Earth's interior such as the Preliminary reference Earth model (PREM).
The Murnaghan equation of state is a relationship between the volume of a body and the pressure to which it is subjected. This is one of many state equations that have been used in earth sciences and shock physics to model the behavior of matter under conditions of high pressure. It owes its name to Francis D. Murnaghan who proposed it in 1944 to reflect material behavior under a pressure range as wide as possible to reflect an experimentally established fact: the more a solid is compressed, the more difficult it is to compress further.

The deep water cycle, or geologic water cycle, involves exchange of water with the mantle, with water carried down by subducting oceanic plates and returning through volcanic activity, distinct from the water cycle process that occurs above and on the surface of Earth. Some of the water makes it all the way to the lower mantle and may even reach the outer core. Mineral physics experiments show that hydrous minerals can carry water deep into the mantle in colder slabs and even "nominally anhydrous minerals" can store several oceans' worth of water.

The lower mantle, historically also known as the mesosphere, represents approximately 56% of Earth's total volume, and is the region from 660 to 2900 km below Earth's surface; between the transition zone and the outer core. The preliminary reference Earth model (PREM) separates the lower mantle into three sections, the uppermost (660–770 km), mid-lower mantle (770–2700 km), and the D layer (2700–2900 km). Pressure and temperature in the lower mantle range from 24–127 GPa and 1900–2600 K. It has been proposed that the composition of the lower mantle is pyrolitic, containing three major phases of bridgmanite, ferropericlase, and calcium-silicate perovskite. The high pressure in the lower mantle has been shown to induce a spin transition of iron-bearing bridgmanite and ferropericlase, which may affect both mantle plume dynamics and lower mantle chemistry.
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust and ends at the top of the lower mantle at 670 km (420 mi). Temperatures range from approximately 500 K at the upper boundary with the crust to approximately 1,200 K at the boundary with the lower mantle. Upper mantle material that has come up onto the surface comprises about 55% olivine, 35% pyroxene, and 5 to 10% of calcium oxide and aluminum oxide minerals such as plagioclase, spinel, or garnet, depending upon depth.

The thermal equation of state is a mathematical expression of pressure, temperature, and, volume. The thermal equation of state for ideal gases is the ideal gas law, expressed as PV=nRT, while the thermal equation of state for solids is expressed as:

















