
The coenzyme Q : cytochrome c – oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain, playing a critical role in biochemical generation of ATP. Complex III is a multisubunit transmembrane protein encoded by both the mitochondrial and the nuclear genomes. Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders. The bc1 complex contains 11 subunits, 3 respiratory subunits, 2 core proteins and 6 low-molecular weight proteins.

Heme, or haem, is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver.

Protoporphyrin ferrochelatase (EC 4.98.1.1, formerly EC 4.99.1.1, or ferrochelatase; systematic name protoheme ferro-lyase (protoporphyrin-forming)) is an enzyme encoded by the FECH gene in humans. Ferrochelatase catalyses the eighth and terminal step in the biosynthesis of heme, converting protoporphyrin IX into heme B. It catalyses the reaction:
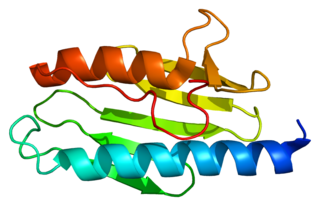
Frataxin is a protein that in humans is encoded by the FXN gene.

Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 in vitro leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

Nuclear respiratory factor 1, also known as Nrf1, Nrf-1, NRF1 and NRF-1, encodes a protein that homodimerizes and functions as a transcription factor which activates the expression of some key metabolic genes regulating cellular growth and nuclear genes required for respiration, heme biosynthesis, and mitochondrial DNA transcription and replication. The protein has also been associated with the regulation of neurite outgrowth. Alternate transcriptional splice variants, which encode the same protein, have been characterized. Additional variants encoding different protein isoforms have been described but they have not been fully characterized. Confusion has occurred in bibliographic databases due to the shared symbol of NRF1 for this gene and for "nuclear factor -like 1" which has an official symbol of NFE2L1.

Transcription regulator protein BACH1 is a protein that in humans is encoded by the BACH1 gene.
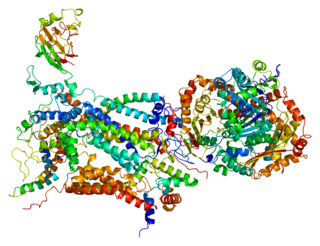
Cytochrome c1, heme protein, mitochondrial (CYC1), also known as UQCR4, MC3DN6, Complex III subunit 4, Cytochrome b-c1 complex subunit 4, or Ubiquinol-cytochrome-c reductase complex cytochrome c1 subunit is a protein that in humans is encoded by the CYC1 gene. CYC1 is a respiratory subunit of Ubiquinol Cytochrome c Reductase, which is located in the inner mitochondrial membrane and is part of the electron transport chain. Mutations in this gene may cause mitochondrial complex III deficiency, nuclear, type 6.

Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the outer membrane of the mitochondria, peroxisomes, and mitochondrial-associated endoplasmic reticulum membrane (MAM). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus.

ATP-binding cassette sub-family B member 7, mitochondrial is a protein that in humans is encoded by the ABCB7 gene.

Succinyl-CoA ligase [ADP-forming] subunit beta, mitochondrial (SUCLA2), also known as ADP-forming succinyl-CoA synthetase (SCS-A), is an enzyme that in humans is encoded by the SUCLA2 gene on chromosome 13.

Fetal and Adult Testis-Expressed 1, encoded by the FATE1 gene in humans, is a protein identified as a cancer-testis antigen (CTA) in hepatocellular carcinomas and gastric and colon cancers. It is testis-specific in the fetus. In adults, it is expressed predominantly in the testis and adrenal glands, with some expression in the lungs, heart, kidneys and throughout the brain.

BCL2-like 13 , also known as BCL2L13 or Bcl-rambo, is a protein which in humans is encoded by the BCL2L13 gene on chromosome 22. This gene encodes a mitochondrially-localized protein which is classified under the Bcl-2 protein family. Overexpression of the encoded protein results in apoptosis. As a result, it has been implicated in cancers such as childhood acute lymphoblastic leukemia (ALL) and glioblastoma multiforme (GBM). Alternatively spliced transcript variants have been observed for this gene, such as Bcl-rambo beta.

Delta-aminolevulinate synthase 2 also known as ALAS2 is a protein that in humans is encoded by the ALAS2 gene. ALAS2 is an aminolevulinic acid synthase.

Delta-aminolevulinate synthase 1 also known as ALAS1 is a protein that in humans is encoded by the ALAS1 gene. ALAS1 is an aminolevulinic acid synthase.

Glutaredoxin 5, also known as GLRX5, is a protein which in humans is encoded by the GLRX5 gene located on chromosome 14. This gene encodes a mitochondrial protein, which is evolutionarily conserved. It is involved in the biogenesis of iron- sulfur clusters, which are required for normal iron homeostasis. Mutations in this gene are associated with autosomal recessive pyridoxine-refractory sideroblastic anemia.

Mitochondrial ferritin is a ferroxidase enzyme that in humans is encoded by the FTMT gene.

In molecular biology mir-451 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
A heme transporter is a protein that delivers heme to the various parts of a biological cell that require it.




















