Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
1/n(AlO
2)−
(SiO
2)
x・yH
2O where Mn+
1/n is either a metal ion or H+. These positive ions can be exchanged for others in a contacting electrolyte solution. H+
exchanged zeolites are particularly useful as solid acid catalysts.

Moonshine is high-proof liquor, traditionally made or distributed illegally. Its clandestine distribution is known as bootlegging. The name was derived from a tradition of creating the alcohol during the nighttime, thereby avoiding detection. In the first decades of the 21st century, commercial distilleries have adopted the term for its outlaw cachet and begun producing their own legally sanctioned, novelty "moonshine", including many flavored varieties, that in some sense continue its tradition, generally having a similar method and/or location of production.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.
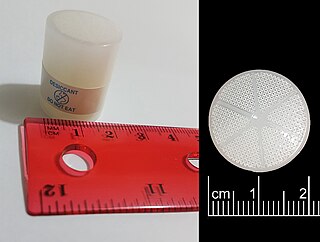
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams.

Activated alumina is manufactured from aluminium hydroxide by dehydroxylating it in a way that produces a highly porous material; this material can have a surface area significantly over 200 m2/g. The compound is used as a desiccant (to keep things dry by adsorbing water from the air) and as a filter of fluoride, arsenic and selenium in drinking water. It is made of aluminium oxide (alumina; Al2O3). It has a very high surface-area-to-weight ratio, due to the many "tunnel like" pores that it has. Activated alumina in its phase composition can be represented only by metastable forms (gamma-Al2O3 etc.). Corundum (alpha-Al2O3), the only stable form of aluminum oxide, does not have such a chemically active surface and is not used as a sorbent.

Sodium aluminate is an inorganic chemical that is used as an effective source of aluminium hydroxide for many industrial and technical applications. Pure sodium aluminate (anhydrous) is a white crystalline solid having a formula variously given as NaAlO2, NaAl(OH)4 (hydrated), Na2O·Al2O3, or Na2Al2O4. Commercial sodium aluminate is available as a solution or a solid.
Other related compounds, sometimes called sodium aluminate, prepared by reaction of Na2O and Al2O3 are Na5AlO4 which contains discrete AlO45− anions, Na7Al3O8 and Na17Al5O16 which contain complex polymeric anions, and NaAl11O17, once mistakenly believed to be β-alumina, a phase of aluminium oxide.
Rectified spirit, also known as neutral spirits, rectified alcohol or ethyl alcohol of agricultural origin, is highly concentrated ethanol that has been purified by means of repeated distillation in a process called rectification. In some countries, denatured alcohol or denatured rectified spirit may commonly be available as "rectified spirit", because in some countries the retail sale of rectified alcohol in its non-denatured form is prohibited.
Sodium aluminosilicate refers to compounds which contain sodium, aluminium, silicon and oxygen, and which may also contain water. These include synthetic amorphous sodium aluminosilicate, a few naturally occurring minerals and synthetic zeolites. Synthetic amorphous sodium aluminosilicate is widely used as a food additive, E 554.

Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperature and significantly differs from the cryogenic distillation commonly used to separate gases. Selective adsorbent materials are used as trapping material, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed gas.

A sorbent is a material that either absorbs or adsorbs liquids or gases.

Nanoporous materials consist of a regular organic or inorganic bulk phase in which a porous structure is present. Nanoporous materials exhibit pore diameters that are most appropriately quantified using units of nanometers. The diameter of pores in nanoporous materials is thus typically 100 nanometers or smaller. Pores may be open or closed, and pore connectivity and void fraction vary considerably, as with other porous materials. Open pores are pores that connect to the surface of the material whereas closed pores are pockets of void space within a bulk material. Open pores are useful for molecular separation techniques, adsorption, and catalysis studies. Closed pores are mainly used in thermal insulators and for structural applications.
Mesoporous silicates are silicates with a special morphology.

Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and imaging. Mesoporous ordered silica films have been also obtained with different pore topologies.

Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIF glasses can be synthesized by the melt-quench method, and the first melt-quenched ZIF glass was firstly made and reported by Bennett et al. back in 2015. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIFs are being investigated for applications such as carbon dioxide capture.

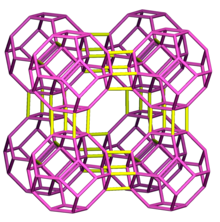
ZSM-5, Zeolite Socony Mobil–5 (framework type MFI from ZSM-5 (five)), is an aluminosilicate zeolite belonging to the pentasil family of zeolites. Its chemical formula is NanAlnSi96–nO192·16H2O (0<n<27). Patented by Mobil Oil Company in 1975, it is widely used in the petroleum industry as a heterogeneous catalyst for hydrocarbon isomerization reactions.
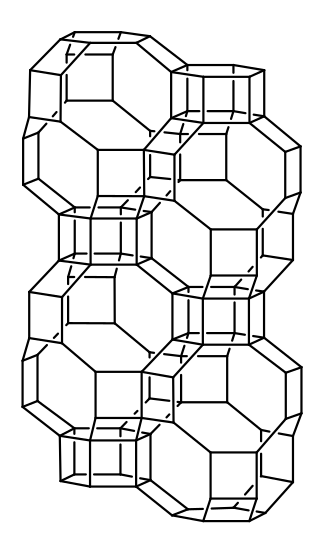
SSZ-13 (framework type code CHA) is a high-silica aluminosilicate zeolite possessing 0.38 × 0.38 nm micropores. It belongs to the ABC-6 family of zeolites as well as offretite, cancrinite, erionite and other related small-pore zeolites. The framework topology is the same as that of chabazite but SSZ-13 has a high silica composition with Si/Al > 5, which leads to low cation exchange capacity. The typical chemical formula of the unit cell can be described as QxNayAl2.4Si33.6O72•zH2O (1.4 < x <27)(0.7 < y < 4.3)(1 < z <7), where Q is N,N,N-1-trimethyladamantammonium. The material was patented by Chevron research Company in 1985, and could potentially be used as a solid catalyst for the methanol-to-olefins (MTO) process and the selective catalytic reduction (SCR) of NOx.
Mesoporous organosilica are a type of silica containing organic groups that give rise to mesoporosity. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts, adsorbents, trapping agents, drug delivery agents, stationary phases in chromatography and chemical sensors.
A zeolite membrane is a synthetic membrane made of crystalline aluminosilicate materials, typically aluminum, silicon, and oxygen with positive counterions such as Na+ and Ca2+ within the structure. Zeolite membranes serve as a low energy separation method. They have recently drawn interest due to their high chemical and thermal stability, and their high selectivity. Currently zeolites have seen applications in gas separation, membrane reactors, water desalination, and solid state batteries. Currently zeolite membranes have yet to be widely implemented commercially due to key issues including low flux, high cost of production, and defects in the crystal structure.