
A mold or mould is one of the structures that certain fungi can form. The dust-like, colored appearance of molds is due to the formation of spores containing fungal secondary metabolites. The spores are the dispersal units of the fungi. Not all fungi form molds. Some fungi form mushrooms; others grow as single cells and are called microfungi.

Zygomycota, or zygote fungi, is a former division or phylum of the kingdom Fungi. The members are now part of two phyla: the Mucoromycota and Zoopagomycota. Approximately 1060 species are known. They are mostly terrestrial in habitat, living in soil or on decaying plant or animal material. Some are parasites of plants, insects, and small animals, while others form symbiotic relationships with plants. Zygomycete hyphae may be coenocytic, forming septa only where gametes are formed or to wall off dead hyphae. Zygomycota is no longer recognised as it was not believed to be truly monophyletic.

Mucor is a microbial genus of approximately 40 species of molds in the family Mucoraceae. Species are commonly found in soil, digestive systems, plant surfaces, some cheeses like Tomme de Savoie, rotten vegetable matter and iron oxide residue in the biosorption process.

Mortierella species are soil fungi belonging to the order Mortierellales within the subphylum Mortierellomycotina. The widespread genus contains about 85 species.

Mucor mucedo, commonly known as the common pinmould, is a fungal plant pathogen and member of the phylum Mucoromycota and the genus Mucor. Commonly found on soil, dung, water, plants and moist foods, Mucor mucedo is a saprotrophic fungus found world-wide with 85 known strains. It is often mistaken for Rhizopus rots on fruits due to similar mould growth shape and colour. Contrastingly, however, Mucor mucedo is found to grow on a wide range of stored grains and plants, including cucumber and tomato. Discovered in Italy in 1729 by P.A. Micheli and later noted by Carl Linnaeus in 1753 in the Species Plantarum, Mucor mucedo was originally classified as Mucor vulgaris by Micheli but later classified synonymous under name Mucor mucedo. The species was redescribed as Ascophora mucedo by H.J. Tode in 1790 but this type resided in a stoloniferous habitat and was later made the type of new genus Rhizopus.

Mucor racemosus is a rapidly growing, weedy mould belonging to the division Mucoromycota. It is one of the earliest fungi to be grown in pure culture and was first isolated in 1886. It has a worldwide distribution and colonizes many habitats such as vegetational products, soil and houses. The fungus is mostly known for its ability to exhibit both filamentous and yeast-like morphologies, often referred to as dimorphism. Stark differences are seen in both forms and conditions of the environment heavily affect the phases of the M. racemosus. Like many fungi, it also reproduces both sexually and asexually. The dimorphic capacity of this species has been proposed as an important factor in its pathogenicity and has enhanced the industrial importance. This species is considered an opportunistic pathogen, generally limited to immunocompromised individuals. It also been associated with allergy and inflammations of facial sinuses. Its association with allergy has made it a common fungus used in allergen medical testing. Industrial use of the fungus is in the production of enzymes and the manufacture of certain dairy foods.

The Zoopagomycotina are a subdivision of the fungal division Zygomycota sensu lato. It contains 5 families and 20 genera. Relationships among and within subphyla of Zygomycota are poorly understood, and their monophyly remains in question, so they are sometimes referred to by the informal name zygomycetes.
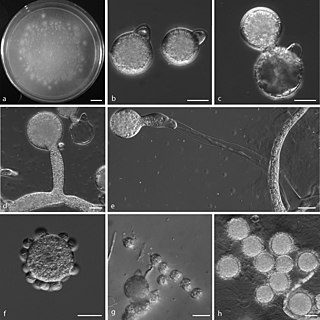
Conidiobolus coronatus is a saprotrophic fungus, first described by Costantin in 1897 as Boudierella coronata. Though this fungus has also been known by the name Entomophthora coronata, the correct name is Conidiobolus coronatus. C. coronatus is able to infect humans and animals, and the first human infection with C. coronatus was reported in Jamaica in 1965.

Spinellus fusiger, commonly known as bonnet mold, is a species of fungus in the phylum Mucoromycota. It is a pin mold that is characterized by erect sporangiophores that are simple in structure, brown or yellowish-brown in color, and with branched aerial filaments that bear the zygospores. It grows as a parasitic mold on mushrooms, including several species from the genera Mycena, including M. haematopus, M. pura, M. epipterygia, M. leptocephala, and various Collybia species, such as C. alkalivirens, C. luteifolia, C. dryophila, and C. butyracea. It has also been found growing on agaric species in Amanita, Gymnopus, and Hygrophorus.

Lichtheimia corymbifera is a thermophilic fungus in the phylum Zygomycota. It normally lives as a saprotrophic mold, but can also be an opportunistic pathogen known to cause pulmonary, CNS, rhinocerebral, or cutaneous infections in animals and humans with impaired immunity.
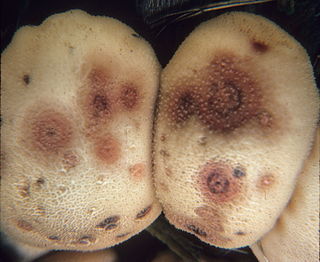
Epicoccum nigrum is a species of fungus in the phylum Ascomycota. A plant pathogen and endophyte, it is a widespread fungus which produces coloured pigments that can be used as antifungal agents against other pathogenic fungi. The fluorescent stain epicocconone is extracted from it.
Cunninghamella bertholletiae is a species of zygomycetous fungi in the order Mucorales. It is found globally, with increased prevalence in Mediterranean and subtropical climates. It typically grows as a saprotroph and is found in a wide variety of substrates, including soil, fruits, vegetables, nuts, crops, and human and animal waste. Although infections are still rare, C. betholletiae is emerging as an opportunistic human pathogen, predominantly in immunocompromised people, leukemia patients, and people with uncontrolled diabetes. Cunninghamella bertholletiae infections are often highly invasive, and can be more difficult to treat with antifungal drugs than infections with other species of the Mucorales, making prompt and accurate recognition and diagnosis of mycoses caused by this fungus an important medical concern.

Rhizopus oryzae is a filamentous heterothallic microfungus that occurs as a saprotroph in soil, dung, and rotting vegetation. This species is very similar to Rhizopus stolonifer, but it can be distinguished by its smaller sporangia and air-dispersed sporangiospores. It differs from R. oligosporus and R. microsporus by its larger columellae and sporangiospores. The many strains of R. oryzae produce a wide range of enzymes such as carbohydrate digesting enzymes and polymers along with a number of organic acids, ethanol and esters giving it useful properties within the food industries, bio-diesel production, and pharmaceutical industries. It is also an opportunistic pathogen of humans causing mucormycosis.

Cunninghamella echinulata is a fungal species in the genus Cunninghamella. It is an asexually reproducing fungus and a mesophile, preferring intermediate temperature ranges. C. echinulata is a common air contaminant, and is currently of interest to the biotechnology industry due to its ability to synthesize γ-linolenic acid as well as its capacity to bioconcentrate metals. This species is a soil saprotroph that forms rhizoids, preferring soils enriched in nitrogen, phosphorus and potassium. It has been reported occasionally an agent of mucormycosis following the inhalation of fungal spores. Czapek's agar is a suitable growth medium for the propagation of C. echinulata.

Aspergillus clavatus is a species of fungus in the genus Aspergillus with conidia dimensions 3–4.5 x 2.5–4.5 μm. It is found in soil and animal manure. The fungus was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.

Rhizopus stolonifer is commonly known as black bread mold. It is a member of Zygomycota and considered the most important species in the genus Rhizopus. It is one of the most common fungi in the world and has a global distribution although it is most commonly found in tropical and subtropical regions. It is a common agent of decomposition of stored foods. Like other members of the genus Rhizopus, R. stolonifer grows rapidly, mostly in indoor environments.

Mucor circinelloides is a dimorphic fungus belonging to the Order Mucorales. It has a worldwide distribution, found mostly in soil, dung and root vegetables. This species is described as not known to be able to produce mycotoxins, however it has been frequently reported to infect animals such as cattle and swine, as well as fowl, platypus and occasionally humans. Ketoacidotic patients are particularly at risk for infection by M. circinelloides.
Cladosporium herbarum is a common fungus found worldwide in organic and inorganic matter. It is efficiently distributed in the air, where it exists as the most frequently occurring fungal species. It can grow over a wide range of temperatures including very cold environments, giving it the ability to grow on refrigerated meat and form "black spots". Its high prevalence in the air and production of allergens makes C. herbarum an important exacerbant of asthma and hay fever.

Mortierella polycephala is a saprotrophic fungus with a wide geographical distribution occurring in many different habitats from soil and plants to salt marshes and slate slopes. It is the type species of the genus Mortierella, and was first described in 1863 by Henri Coemans. A characteristic feature of the fungus is the presence of stylospores, which are aerial, spiny resting spores (chlamydospores).

Mucoromycota is a division within the kingdom fungi. It includes a diverse group of various molds, including the common bread molds Mucor and Rhizopus. It is a sister phylum to Dikarya.
















