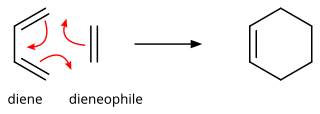
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:

In organic chemistry, the ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond, in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.

Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints.

Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.
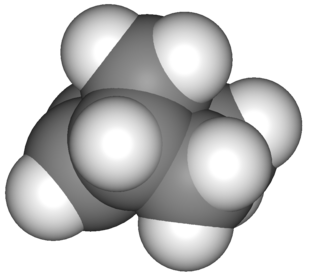
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.

Chlorendic acid, or 1,4,5,6,7,7-hexachlorobicyclo[2.2.1]-hept-5-ene-2,3-dicarboxylic acid, is a chlorinated carboxylic acid used in the synthesis of some flame retardants and polymers. It is a common breakdown product of several organochlorine insecticides.
Barrelene is a bicyclic organic compound with chemical formula C8H8 and systematic name bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by Howard Zimmerman in 1960, the name derives from the resemblance to a barrel, with the staves being three ethylene units attached to two methine groups. It is the formal Diels–Alder adduct of benzene and acetylene. Due to its unusual molecular geometry, the compound is of considerable interest to theoretical chemists.
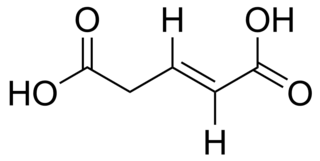
trans-Glutaconic acid is an organic compound with formula HO2CCH=CHCH2CO2H. This dicarboxylic acid exists as a colorless solid and is related to the saturated chemical glutaric acid, HO2CC(CH2)3CO2H. Esters and salts of glutaconic acid are called glutaconates.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and the dienophile are both part of the same molecule. The reaction leads to the formation of the cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction can gives rise to various natural derivatives of decalin.
Chiral Lewis acids (CLAs) are a type of Lewis acid catalyst. These acids affect the chirality of the substrate as they react with it. In such reactions, synthesis favors the formation of a specific enantiomer or diastereomer. The method is an enantioselective asymmetric synthesis reaction. Since they affect chirality, they produce optically active products from optically inactive or mixed starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically a metal, such as indium, zinc, lithium, aluminium, titanium, or boron. The chiral-altering ligands employed for synthesizing these acids often have multiple Lewis basic sites that allow the formation of a ring structure involving the metal atom.

Cycloprop-2-ene carboxylic acid is a mycotoxin found in some mushrooms such as Russula subnigricans.
The retro-Diels–Alder reaction is the reverse of the Diels–Alder (DA) reaction, a [4+2] cycloelimination. It involves the formation of a diene and dienophile from a cyclohexene. It can be accomplished spontaneously with heat, or with acid or base mediation.
Cycloisomerization is any isomerization in which the cyclic isomer of the substrate is produced in the reaction coordinate. The greatest advantage of cycloisomerization reactions is its atom economical nature, by design nothing is wasted, as every atom in the starting material is present in the product. In most cases these reactions are mediated by a transition metal catalyst, in few cases organocatalysts and rarely do they occur under thermal conditions. These cyclizations are able to be performed with excellent levels of selectivity in numerous cases and have transformed cycloisomerization into a powerful tool for unique and complex molecular construction. Cycloisomerization is a very broad topic in organic synthesis and many reactions that would be categorized as such exist. Two basic classes of these reactions are intramolecular Michael addition and Intramolecular Diels–Alder reactions. Under the umbrella of cycloisomerization, enyne and related olefin cycloisomerizations are the most widely used and studied reactions.
In organosulfur chemistry, the thiol-ene reaction is an organic reaction between a thiol and an alkene to form a thioether. This reaction was first reported in 1905, but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications. This reaction is accepted as a click chemistry reaction given the reactions' high yield, stereoselectivity, high rate, and thermodynamic driving force.

Vinyl norbornene (VNB) is an organic compound that consists of a vinyl group attached to norbornene. It is a colorless liquid. The compound exists as endo and exo isomers, but these are not typically separated. It is an intermediate in the production of the commercial polymer EPDM. It is prepared by the Diels-Alder reaction of butadiene and cyclopentadiene.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.
Tetrahydrophthalic anhydride is an organic compound with the formula C6H8C2O3. The compound exists as two isomers, this article being focused on the more common cis isomer. It is a precursor to other compounds including the dicarboxylic acid tetrahydrophthalic acid as well the tetrahydrophthalimide, which is a precursor to the fungicide Captan. It is a white solid that is soluble in organic solvents.
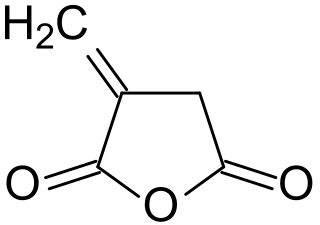
Itaconic anhydride is the cyclic anhydride of itaconic acid and is obtained by the pyrolysis of citric acid. It is a colourless, crystalline solid, which dissolves in many polar organic solvents and hydrolyzes forming itaconic acid. Itaconic anhydride and its derivative itaconic acid have been promoted as biobased "platform chemicals" and bio- building blocks.) These expectations, however, have not been fulfilled.


















