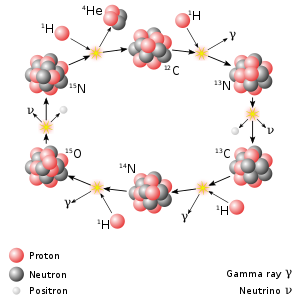Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino. Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.

TRIUMF is Canada's national particle accelerator centre. It is considered Canada's premier physics laboratory, and consistently regarded as one of the world's leading subatomic physics research centres. Owned and operated by a consortium of universities, it is on the south campus of one of its founding members, the University of British Columbia in Vancouver, British Columbia, Canada. It houses the world's largest normal conducting cyclotron, a source of 520 MeV protons, which was named an IEEE Milestone in 2010. Its accelerator-focused activities involve particle physics, nuclear physics, nuclear medicine, materials science, and detector and accelerator development.
Technetium (43Tc) is one of the two elements with Z < 83 that have no stable isotopes; the other such element is promethium. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc, 98Tc, and 99Tc.
Natural gallium (31Ga) consists of a mixture of two stable isotopes: gallium-69 and gallium-71. Twenty-nine radioisotopes are known, all synthetic, with atomic masses ranging from 56 to 86; along with three nuclear isomers, 64mGa, 72mGa and 74mGa. Most of the isotopes with atomic mass numbers below 69 decay to isotopes of zinc, while most of the isotopes with masses above 71 decay to isotopes of germanium. Among them, the most commercially important radioisotopes are gallium-67 and gallium-68.
There are three known stable isotopes of oxygen (8O): 16
O
, 17
O
, and 18
O
.
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Thirteen radioisotopes are also known, with atomic masses ranging from 9 to 23, along with three nuclear isomers. All of these radioisotopes are short-lived, the longest-lived being nitrogen-13 with a half-life of 9.965(4) min. All of the others have half-lives below 7.15 seconds, with most of these being below 620 milliseconds. Most of the isotopes with atomic mass numbers below 14 decay to isotopes of carbon, while most of the isotopes with masses above 15 decay to isotopes of oxygen. The shortest-lived known isotope is nitrogen-10, with a half-life of 143(36) yoctoseconds, though the half-life of nitrogen-9 has not been measured exactly.
Carbon (6C) has 15 known isotopes, from 8
C
to 22
C
, of which 12
C
and 13
C
are stable. The longest-lived radioisotope is 14
C
, with a half-life of 5.70(3)×103 years. This is also the only carbon radioisotope found in nature, as trace quantities are formed cosmogenically by the reaction 14
N
+
n
→ 14
C
+ 1
H
. The most stable artificial radioisotope is 11
C
, which has a half-life of 20.3402(53) min. All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is 8
C
, with a half-life of 3.5(1.4)×10−21 s. Light isotopes tend to decay into isotopes of boron and heavy ones tend to decay into isotopes of nitrogen.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.
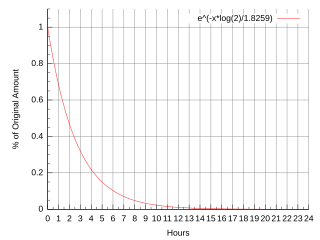
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96% of the time and electron capture 4% of the time. Both modes of decay yield stable oxygen-18.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).
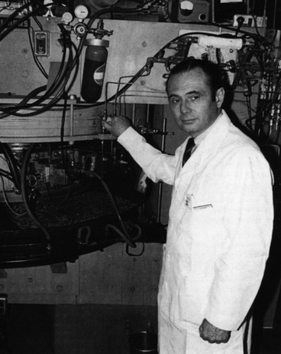
Michel Matthew Ter-Pogossian was an American medical physicist. He was professor of radiology at the Washington University School of Medicine for over 30 years. A pioneer in nuclear medicine, he is best known for his research on the positron emission tomography (PET). He is considered one of its creators and often referred to as the "father of PET."
Copper-64 (64Cu) is a positron and beta emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography. Its unusually long half-life (12.7-hours) for a positron-emitting isotope makes it increasingly useful when attached to various ligands, for PET and PET-CT scanning.
Cardiac PET is a form of diagnostic imaging in which the presence of heart disease is evaluated using a PET scanner. Intravenous injection of a radiotracer is performed as part of the scan. Commonly used radiotracers are Rubidium-82, Nitrogen-13 ammonia and Oxygen-15 water.
Advanced Cyclotron Systems, Inc. (ACSI) is a company based in Richmond, British Columbia, Canada that supplies and services cyclotrons predominantly used for the production of medical isotopes by hospitals for nuclear medicine. The company was a spin-off of the research program at TRIUMF. The machines are used for the production of isotopes used in Positron emission tomography (PET), Single-photon emission computed tomography (SPECT) or production of technetium-99 for molecular imaging. ACSI controls approximately half the world market for such machines,
Radiofluorination is the process by which a radioactive isotope of fluorine is attached to a molecule and is preferably performed by nucleophilic substitution using nitro or halogens as leaving groups. Fluorine-18 is the most common isotope used for this procedure. This is due to its 97% positron emission and relatively long 109.8 min half-life. The half-life allows for a long enough time to be incorporated into the molecule and be used without causing exceedingly harmful effects. This process has many applications especially with the use of positron emission tomography (PET) as the aforementioned low positron energy is able to yield a high resolution in PET imaging.

Oxygen-15 labelled water (also known as 15O-water, [O-15]-H2O, or H215O) is a radioactive variation of regular water, in which the oxygen atom has been replaced by oxygen-15 (15O), a positron-emitting isotope. 15O-water is used as a radioactive tracer for measuring and quantifying blood flow using positron emission tomography (PET) in the heart, brain and tumors.
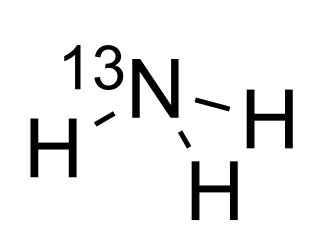
Ammonia (13N) is a medication for diagnostic positron emission tomography (PET) imaging of the myocardium.

CERN-MEDical Isotopes Collected from ISOLDE (MEDICIS) is a facility located in the Isotope Separator Online DEvice (ISOLDE) facility at CERN, designed to produce high-purity isotopes for developing the practice of patient diagnosis and treatment. The facility was initiated in 2010, with its first radioisotopes (terbium-155) produced on 12 December 2017.