Related Research Articles

A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effects, or adversely affects the usefulness of a resource. These can be both naturally forming or anthropogenic in origin. Pollutants result in environmental pollution or become public health concerns when they reach a concentration high enough to have significant negative impacts.

Smog, or smoke fog, is a type of intense air pollution. The word "smog" was coined in the early 20th century, and is a portmanteau of the words smoke and fog to refer to smoky fog due to its opacity, and odor. The word was then intended to refer to what was sometimes known as pea soup fog, a familiar and serious problem in London from the 19th century to the mid-20th century. This kind of visible air pollution is composed of nitrogen oxides, sulfur oxide, ozone, smoke and other particulates. Man-made smog is derived from coal combustion emissions, vehicular emissions, industrial emissions, forest and agricultural fires and photochemical reactions of these emissions.

Smoke is a collection of airborne particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product of fires, but may also be used for pest control (fumigation), communication, defensive and offensive capabilities in the military, cooking, or smoking. It is used in rituals where incense, sage, or resin is burned to produce a smell for spiritual or magical purposes. It can also be a flavoring agent and preservative.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort, and well-being of building occupants. Poor indoor air quality has been linked to sick building syndrome, reduced productivity, and impaired learning in schools. Common pollutants of indoor air include: Secondhand tobacco smoke, air pollutants from indoor combustion, radon, molds and other allergens, carbon monoxide, volatile organic compounds, legionella and other bacteria, asbestos fibers, carbon dioxide, ozone and particulates. Source control, filtration, and the use of ventilation to dilute contaminants are the primary methods for improving indoor air quality in most buildings.
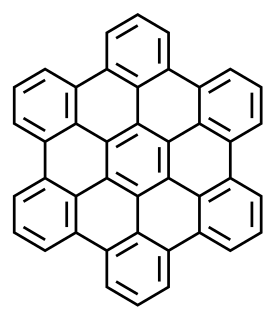
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals is naphthalene, having two aromatic rings, and the three-ring compounds are anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.
Volatile organic compounds (VOCs) are organic chemicals that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility.

Diesel exhaust is the gaseous exhaust produced by a diesel type of internal combustion engine, plus any contained particulates. Its composition may vary with the fuel type or rate of consumption, or speed of engine operation, and whether the engine is in an on-road vehicle, farm vehicle, locomotive, marine vessel, or stationary generator or other application.
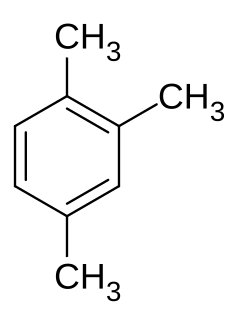
Non-methane volatile organic compounds (NMVOCs) are a set of organic compounds that are typically photochemically reactive in the atmosphere—marked by the exclusion of methane. NMVOCs include a large variety of chemically different compounds, such as benzene, ethanol, formaldehyde, cyclohexane, 1,1,1-trichloroethane and acetone. Essentially, NMVOCs are identical to volatile organic compounds (VOCs), but with methane excluded. Methane is excluded in air-pollution contexts because it is not toxic. It is however a very potent greenhouse gas, with low reactivity and thus a long lifetime in the atmosphere. An important subset of NMVOCs are the non-methane hydrocarbons (NMHCs).
Air pollution dispersion – distribution of air pollution into the atmosphere. Air pollution is the introduction of particulates, biological molecules, or other harmful materials into Earth's atmosphere, causing disease, death to humans, damage to other living organisms such as food crops, or the natural or built environment. Air pollution may come from anthropogenic or natural sources. Dispersion refers to what happens to the pollution during and after its introduction; understanding this may help in identifying and controlling it. Air pollution dispersion has become the focus of environmental conservationists and governmental environmental protection agencies of many countries regarding air pollution control.

Air pollution is the contamination of air due to the presence of substances in the atmosphere that are harmful to the health of humans and other living beings, or cause damage to the climate or to materials. There are many different types of air pollutants, such as gases, particulates, and biological molecules. Air pollution can cause diseases, allergies, and even death to humans; it can also cause harm to other living organisms such as animals and food crops, and may damage the natural environment or built environment. Air pollution can be caused by both human activities and natural phenomena.
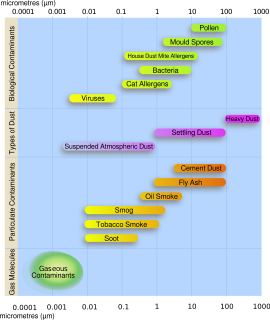
Particulates – also known as atmospheric aerosol particles, atmospheric particulate matter, particulate matter (PM) or suspended particulate matter (SPM) – are microscopic particles of solid or liquid matter suspended in the air. The term aerosol commonly refers to the particulate/air mixture, as opposed to the particulate matter alone. Sources of particulate matter can be natural or anthropogenic. They have impacts on climate and precipitation that adversely affect human health, in ways additional to direct inhalation.

Air pollution in India is a serious environmental issue. Of the 30 most polluted cities in the world, 21 were in India in 2019. As per a study based on 2016 data, at least 140 million people in India breathe air that is 10 times or more over the WHO safe limit and 13 of the world's 20 cities with the highest annual levels of air pollution are in India. 51% of the pollution is caused by industrial pollution, 27 % by vehicles, 17% by crop burning and 5% by other sources. Air pollution contributes to the premature deaths of 2 million Indians every year. Emissions come from vehicles and industry, whereas in rural areas, much of the pollution stems from biomass burning for cooking and keeping warm. In autumn and spring months, large scale crop residue burning in agriculture fields – a cheaper alternative to mechanical tilling – is a major source of smoke, smog and particulate pollution. India has a low per capita emissions of greenhouse gases but the country as a whole is the third largest greenhouse gas producer after China and the United States. A 2013 study on non-smokers has found that Indians have 30% weaker lung function than Europeans.

An aethalometer is an instrument for measuring the concentration of optically absorbing (‘black’) suspended particulates in a gas colloid stream; commonly visualized as smoke or haze, often seen in ambient air under polluted conditions. The word aethalometer is derived from the Classical Greek verb aethaloun, meaning "to blacken with soot".

Aerosol mass spectrometry is the application of mass spectrometry to the analysis of the composition of aerosol particles. Aerosol particles are defined as solid and liquid particles suspended in a gas (air), with size range of 3 nm to 100 μm in diameter and are produced from natural and anthropogenic sources, through a variety of different processes that include wind-blown suspension and combustion of fossil fuels and biomass. Analysis of these particles is important owing to their major impacts on global climate change, visibility, regional air pollution and human health. Aerosols are very complex in structure, can contain thousands of different chemical compounds within a single particle, and need to be analysed for both size and chemical composition, in real-time or off-line applications.
Air pollution measurement is the process of collecting and measuring the components of air pollution, notably gases and particulates. The earliest devices used to measure pollution include rain gauges, Ringelmann charts for measuring smoke, and simple soot and dust collectors known as deposit gauges. Modern air pollution measurement is largely automated and carried out using many different devices and techniques. These range from simple absorbent test tubes known as diffusion tubes through to highly sophisticated chemical and physical sensors that give almost real-time pollution measurements, which are used to generate air quality indexes.

In an oil and gas production, flash-gas is a spontaneous vapor that is produced from the heating or depressurization of the extracted oil mixture during different phases of production. Flash evaporation, or flashing, is the process of volatile components suddenly vaporizing from their liquid state. This often happens during the transportation of petroleum products through pipelines and into vessels, such as when the stream from a common separation unit flows into an on-site atmospheric storage tank. Vessels that are used to intentionally “flash” a mixture of gas and saturated liquids are aptly named "flash drums." A type of vapor-liquid separator. A venting apparatus is used in these vessels to prevent damage due to increasing pressure, extreme cases of this are referred to as boiling liquid expanding vapor explosion (BLEVE).
Kimberly A. Prather is an American scientist who is an Atmospheric Chemist, Distinguished Chair in Atmospheric Chemistry, and a Distinguished Professor at the Scripps Institution of Oceanography and Department of Chemistry and Biochemistry at UC San Diego. Her work focuses on how humans are influencing the atmosphere and climate. In 2019, she was elected a member of the National Academy of Engineering for technologies that transformed understanding of aerosols and their impacts on air quality, climate, and human health. In 2020, she was elected as a member of the National Academy of Sciences. She is also an elected Fellow of the American Geophysical Union, the American Association for the Advancement of Science, and the American Academy of Arts and Sciences.
Wood smoke is a major source of air pollution, especially particulate pollution,pollution by polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) such as formaldehyde.
Particulate pollution is pollution of an environment that consists of particles suspended in some medium. There are three primary forms: atmospheric particulate matter, marine debris, and space debris. Some particles are released directly from a specific source, while others form in chemical reactions in the atmosphere. Particulate pollution can be derived from either natural sources or anthropogenic processes.
References
- ↑ Pope, C Arden; et al. (2002). "Cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution". J. Am. Med. Assoc. 287 (9): 1132–1141. doi:10.1001/jama.287.9.1132. PMC 4037163 . PMID 11879110.
- ↑ Cass, Glen R (1998). "Organic molecular tracers for particulate air pollution sources". TrAC Trends in Analytical Chemistry. 17 (6): 356–366. doi:10.1016/S0165-9936(98)00040-5.
- ↑ H Seinfeld and N. Pandis. Atmospheric chemistry and physics: from air pollution to climate change. (2006)
- ↑ Rogge, Wolfgang F.; Lynn M. Hildemann; Monica A. Mazurek; Glen R. Cass; Bernd R. T. Simoneit (1993). "Sources of fine organic aerosol 1-9". Environmental Science and Technology. 27 (13): 2700–2711. Bibcode:1993EnST...27.2700R. doi:10.1021/es00049a008.
- ↑ Schauer, James J.; Michael J. Kleeman; Glen R. Cass; Bernd R. T. Simoneit (1999). "Measurement of Emissions from Air Pollution Sources. 1-5". Environmental Science and Technology. 36 (6): 1169–1180. Bibcode:2002EnST...36.1169S. doi:10.1021/es0108077. PMID 11944666.