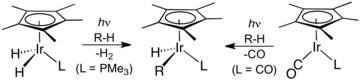
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination.

Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula [RhCl(PPh3)3], where 'Ph' denotes a phenyl group). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

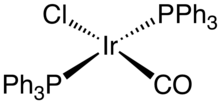
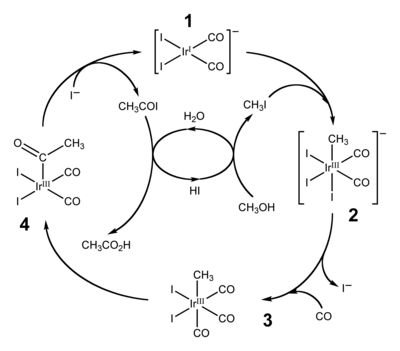
Vaska's complex is the trivial name for the chemical compound trans-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
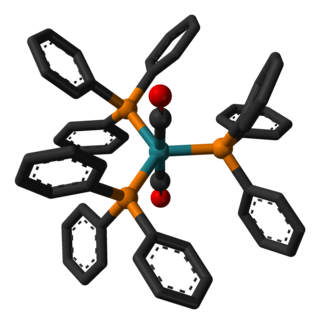
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center.

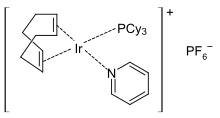
Crabtree's catalyst is an organoiridium compound with the formula [C8H12IrP(C6H11)3C5H5N]PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group.
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Organocobalt chemistry is the chemistry of organometallic compounds containing a carbon to cobalt chemical bond. Organocobalt compounds are involved in several organic reactions and the important biomolecule vitamin B12 has a cobalt-carbon bond. Many organocobalt compounds exhibit useful catalytic properties, the preeminent example being dicobalt octacarbonyl.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.

Cyclooctadiene iridium chloride dimer is an organoiridium compound with the formula [Ir(μ2-Cl)(COD)]2, where COD is the diene 1,5-cyclooctadiene (C8H12). It is an orange-red solid that is soluble in organic solvents. The complex is used as a precursor to other iridium complexes, some of which are used in homogeneous catalysis. The solid is air-stable but its solutions degrade in air.

Chlorobis(cyclooctene)iridium dimer is an organoiridium compound with the formula Ir2Cl2(C8H14)4, where C8H14 is cis-cyclooctene. Sometimes abbreviated Ir2Cl2(coe)4, it is a yellow, air-sensitive solid that is used as a precursor to many other organoiridium compounds and catalysts.

Transition metal acyl complexes describes organometallic complexes containing one or more acyl (RCO) ligands. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.
Iridium compounds are compounds containing the element iridium (Ir). Iridium forms compounds in oxidation states between −3 and +9, but the most common oxidation states are +1, +3, and +4. Well-characterized compounds containing iridium in the +6 oxidation state include IrF6 and the oxides Sr2MgIrO6 and Sr2CaIrO6. iridium(VIII) oxide was generated under matrix isolation conditions at 6 K in argon. The highest oxidation state (+9), which is also the highest recorded for any element, is found in gaseous [IrO4]+.