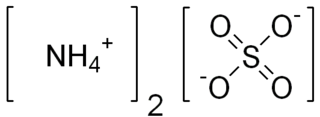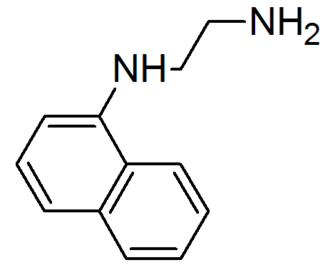Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the concentration of an identified analyte. Since volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the titrant or titrator is prepared as a standard solution. A known concentration and volume of titrant reacts with a solution of analyte or titrand to determine concentration. The volume of titrant reacted is called titration volume.

Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible spectral regions. This means it uses light in the visible and adjacent ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. Absorption spectroscopy is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate and copper(II) sulphate pentahydrate., often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive reaction. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to a brick-red precipitate.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
In environmental chemistry, the chemical oxygen demand (COD) is an indicative measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is commonly expressed in mass of oxygen consumed over volume of solution which in SI units is milligrams per litre (mg/L). A COD test can be used to easily quantify the amount of organics in water. The most common application of COD is in quantifying the amount of oxidizable pollutants found in surface water or wastewater. COD is useful in terms of water quality by providing a metric to determine the effect an effluent will have on the receiving body, much like biochemical oxygen demand (BOD).

Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte based on its mass. The principle behind this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
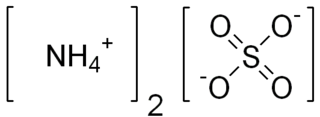
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.

Tetraamminecopper(II) sulfate is the inorganic compound with the formula [Cu(NH3)4(H2O)n]SO4. This dark blue solid is a metal complex with faint odour of ammonia. It is closely related to Schweizer's reagent, which is used for the production of cellulose fibers in the production of rayon. It is used to print fabrics, used as a pesticide and to make other copper compounds like copper nano-powder.
Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point.

The biuret test, also known as Piotrowski's test, is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides, a copper(II) ion forms mauve-colored coordination complexes in an alkaline solution. Several variants on the test have been developed, such as the BCA test and the Modified Lowry test.
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.

Wet chemistry is a form of analytical chemistry that uses classical methods such as observation to analyze materials. It is called wet chemistry since most analyzing is done in the liquid phase. Wet chemistry is also called bench chemistry since many tests are performed at lab benches.
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance(s) present in a sample.

Molybdenum blue is a term applied to:
In analytical chemistry, argentometry is a type of titration involving the silver(I) ion. Typically, it is used to determine the amount of chloride present in a sample. The sample solution is titrated against a solution of silver nitrate of known concentration. Chloride ions react with silver(I) ions to give the insoluble silver chloride:
A methylene blue active substances assay, or MBAS assay, is a colorimetric analysis test method that uses methylene blue to detect the presence of anionic surfactants in a sample of water. An anionic surfactant detected by the color reaction is called a methylene blue active substance (MBAS).
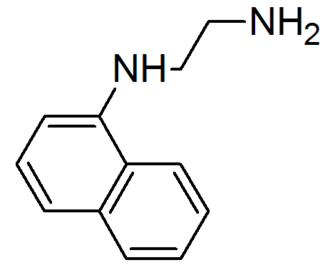
N-(1-Naphthyl)ethylenediamine, also called a 'Griess reagent' is an organic compound. It is commercially available and finds application in quantitative inorganic analysis of nitrates, nitrite and sulfonamide in blood, using Griess test.