In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans. [5] It is implicated in the development and progression of melanomas. [6]
In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans. [5] It is implicated in the development and progression of melanomas. [6]
PDPK1 is a master kinase, which is crucial for the activation of AKT/PKB and many other AGC kinases including PKC, S6K, SGK. An important role for PDPK1 is in the signaling pathways activated by several growth factors and hormones including insulin signaling.
Mice lacking PDPK1 die during early embryonic development, indicating that this enzyme is critical for transmitting the growth-promoting signals necessary for normal mammalian development.
Mice that are deficient in PDPK1 have a ≈40% decrease in body mass, mild glucose intolerance, and are resistant to cancer brought about by hyperactivation of the PI3K pathway (PTEN+/-). [7] [8]
Plant PDK1 plays an important role in regulating PIN-mediated auxin transport, and is thus involved in various developmental processes, such as embryonic development, lateral root formation, vasculature patterning, apical hook formation, gravitropism and phototropism. [9]
PDPK1 stands for 3-phosphoinositide-dependent protein kinase 1. PDPK1 functions downstream of PI3K through PDPK1's interaction with membrane phospholipids including phosphatidylinositols, phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate. PI3K indirectly regulates PDPK1 by phosphorylating phosphatidylinositols which in turn generates phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate. However, PDPK1 is believed to be constitutively active and does not always require phosphatidylinositols for its activities.
Phosphatidylinositols are only required for the activation at the membrane of some substrates including AKT. PDPK1 however does not require membrane lipid binding for the efficient phosphorylation of most of its substrates in the cytosol (not at the cell membrane).
The structure of PDPK1 can be divided into two domains; the kinase or catalytic domain and the PH domain. The PH domain functions mainly in the interaction of PDPK1 with phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate which is important in localization and activation of some of membrane associated PDPK1's substrates including AKT.
The kinase domain has three ligand binding sites; the substrate binding site, the ATP binding site, and the docking site (also known as PIF pocket). Several PDPK1 substrates including S6K and Protein kinase C, require the binding at this docking site. Small molecule allosteric activators of PDPK1 were shown to selectively inhibit activation of substrates that require docking site interaction. These compounds do not bind to the active site and allow PDPK1 to activate other substrates that do not require docking site interaction. PDPK1 is constitutively active and at present, there is no known inhibitor proteins for PDPK1.
The activation of PDPK1's main effector, AKT, is believed to require a proper orientation of the kinase and PH domains of PDPK1 and AKT at the membrane.
Phosphoinositide-dependent kinase-1 has been shown to interact with:
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades.

Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.
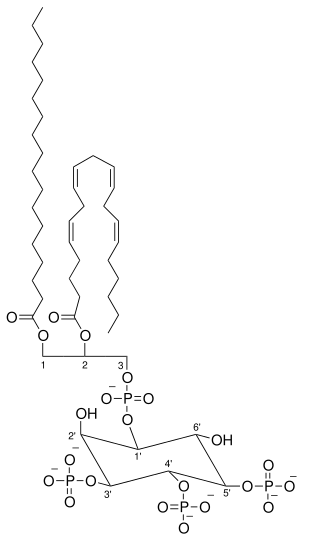
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.
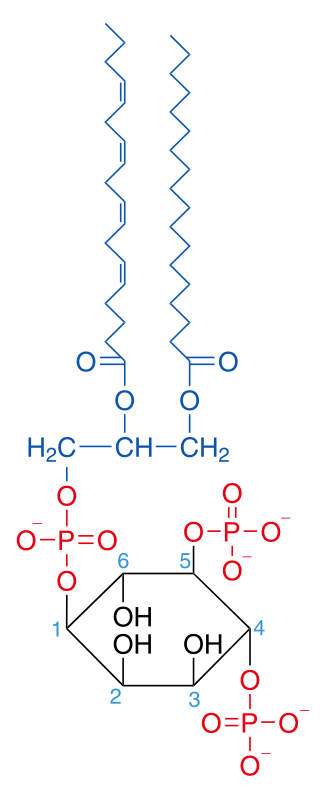
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

Protein kinase C, zeta (PKCζ), also known as PRKCZ, is a protein in humans that is encoded by the PRKCZ gene. The PRKCZ gene encodes at least two alternative transcripts, the full-length PKCζ and an N-terminal truncated form PKMζ. PKMζ is thought to be responsible for maintaining long-term memories in the brain. The importance of PKCζ in the creation and maintenance of long-term potentiation was first described by Todd Sacktor and his colleagues at the SUNY Downstate Medical Center in 1993.

RAC(Rho family)-alpha serine/threonine-protein kinase is an enzyme that in humans is encoded by the AKT1 gene. This enzyme belongs to the AKT subfamily of serine/threonine kinases that contain SH2 protein domains. It is commonly referred to as PKB, or by both names as "Akt/PKB".

Phosphatidylinositol 3-kinase regulatory subunit alpha is an enzyme that in humans is encoded by the PIK3R1 gene.

AKT2, also known as RAC-beta serine/threonine-protein kinase, is an enzyme that in humans is encoded by the AKT2 gene. It influences metabolite storage as part of the insulin signal transduction pathway.

RAC-gamma serine/threonine-protein kinase is an enzyme that in humans is encoded by the AKT3 gene.

Serine/threonine-protein kinase N1 is an enzyme that in humans is encoded by the PKN1 gene.

Ribosomal protein S6 kinase alpha-2 is an enzyme that in humans is encoded by the RPS6KA2 gene.

Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing alpha polypeptide is an enzyme that in humans is encoded by the PIK3C2A gene.

Serine/threonine-protein kinase N2 is an enzyme that in humans and Strongylocentrotus purpuratus is encoded by the PKN2 gene.

Ras GTPase-activating protein 3 is an enzyme that in humans is encoded by the RASA3 gene.

Pleckstrin homology domain-containing family A member 1 is a protein that in humans is encoded by the PLEKHA1 gene.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

BIM-1 and the related compounds BIM-2, BIM-3, and BIM-8 are bisindolylmaleimide-based protein kinase C (PKC) inhibitors. These inhibitors also inhibit PDK1 explaining the higher inhibitory potential of LY33331 compared to the other BIM compounds a bisindolylmaleimide inhibitor toward PDK1.
Leonard (Len) R Stephens FRS is a molecular biologist, senior group leader and associate director at the Babraham Institute.