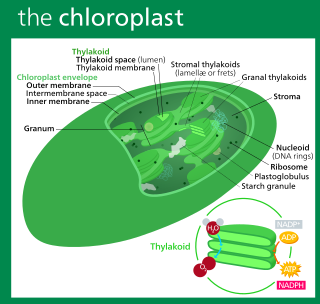
A chloroplast is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like Arabidopsis and wheat.

Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their activities. Photosynthetic organisms use intracellular organic compounds to store the chemical energy they produce in photosynthesis within organic compounds like sugars, glycogen, cellulose and starches. Photosynthesis is usually used to refer to oxygenic photosynthesis, a process that produces oxygen. To use this stored chemical energy, the organisms' cells metabolize the organic compounds through another process called cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.

Arabidopsis thaliana, the thale cress, mouse-ear cress or arabidopsis, is a small plant from the mustard family (Brassicaceae), native to Eurasia and Africa. Commonly found along the shoulders of roads and in disturbed land, it is generally considered a weed.
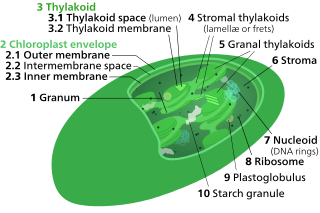
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.
Photobiology is the scientific study of the beneficial and harmful interactions of light in living organisms. The field includes the study of photophysics, photochemistry, photosynthesis, photomorphogenesis, visual processing, circadian rhythms, photomovement, bioluminescence, and ultraviolet radiation effects.
In developmental biology, photomorphogenesis is light-mediated development, where plant growth patterns respond to the light spectrum. This is a completely separate process from photosynthesis where light is used as a source of energy. Phytochromes, cryptochromes, and phototropins are photochromic sensory receptors that restrict the photomorphogenic effect of light to the UV-A, UV-B, blue, and red portions of the electromagnetic spectrum.
Shade avoidance is a set of responses that plants display when they are subjected to the shade of another plant. It often includes elongation, altered flowering time, increased apical dominance and altered partitioning of resources. This set of responses is collectively called the shade-avoidance syndrome (SAS).
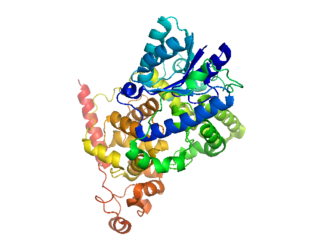
Cryptochromes are a class of flavoproteins found in plants and animals that are sensitive to blue light. They are involved in the circadian rhythms and the sensing of magnetic fields in a number of species. The name cryptochrome was proposed as a portmanteau combining the chromatic nature of the photoreceptor, and the cryptogamic organisms on which many blue-light studies were carried out.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells become turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.

The eyespot apparatus is a photoreceptive organelle found in the flagellate or (motile) cells of green algae and other unicellular photosynthetic organisms such as euglenids. It allows the cells to sense light direction and intensity and respond to it, prompting the organism to either swim towards the light, or away from it. A related response occurs when cells are briefly exposed to high light intensity, causing the cell to stop, briefly swim backwards, then change swimming direction. Eyespot-mediated light perception helps the cells in finding an environment with optimal light conditions for photosynthesis. Eyespots are the simplest and most common "eyes" found in nature, composed of photoreceptors and areas of bright orange-red red pigment granules. Signals relayed from the eyespot photoreceptors result in alteration of the beating pattern of the flagella, generating a phototactic response.

A pulvinus is a joint-like thickening at the base of a plant leaf or leaflet that facilitates growth-independent movement. Pulvini are common, for example, in members of the bean family Fabaceae (Leguminosae) and the prayer plant family Marantaceae.

In biology, phototropism is the growth of an organism in response to a light stimulus. Phototropism is most often observed in plants, but can also occur in other organisms such as fungi. The cells on the plant that are farthest from the light contain a hormone called auxin that reacts when phototropism occurs. This causes the plant to have elongated cells on the furthest side from the light. Phototropism is one of the many plant tropisms, or movements, which respond to external stimuli. Growth towards a light source is called positive phototropism, while growth away from light is called negative phototropism. Negative phototropism is not to be confused with skototropism, which is defined as the growth towards darkness, whereas negative phototropism can refer to either the growth away from a light source or towards the darkness. Most plant shoots exhibit positive phototropism, and rearrange their chloroplasts in the leaves to maximize photosynthetic energy and promote growth. Some vine shoot tips exhibit negative phototropism, which allows them to grow towards dark, solid objects and climb them. The combination of phototropism and gravitropism allow plants to grow in the correct direction.
A Light-oxygen-voltage-sensing domain is a protein sensor used by a large variety of higher plants, microalgae, fungi and bacteria to sense environmental conditions. In higher plants, they are used to control phototropism, chloroplast relocation, and stomatal opening, whereas in fungal organisms, they are used for adjusting the circadian temporal organization of the cells to the daily and seasonal periods. They are a subset of PAS domains.

Joanne Chory is an American plant biologist and geneticist. Chory is a professor and director of the Plant Molecular and Cellular Biology Laboratory, at the Salk Institute for Biological Studies and an investigator of the Howard Hughes Medical Institute.
LiMETER stands for light-inducible membrane-tethered peripheral endoplasmic reticulum (ER). LiMETER is an optogenetics tool designed to reversibly label cortical ER or the apposition between plasma membrane (PM) and endoplasmic reticulum (ER) membranes.
As a model organism, the Arabidopsis thaliana response to salinity is studied to aid understanding of other more economically important crops.

Anthony R. Cashmore is a biochemist and plant molecular biologist, best known for identifying cryptochrome photoreceptor proteins. These specialized proteins are critical for plant development and play an essential role in circadian rhythms of plants and animals. A Professor emeritus in the Department of Biology at the University of Pennsylvania, Cashmore led the Plant Science Institute from the time of his appointment in 1986 until his retirement in 2011. He was elected to the National Academy of Sciences in 2003.
Plant nucleus movement is the movement of the cell nucleus in plants by the cytoskeleton.
Christoph Benning is a German–American plant biologist. He is an MSU Foundation Professor and University Distinguished Professor at Michigan State University. Benning's research into lipid metabolism in plants, algae and photosynthetic bacteria, led him to be named Editor-in-Chief of The Plant Journal in October 2008.











