
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, magnetism is one of two aspects of electromagnetism.

Organic electronics is a field of materials science concerning the design, synthesis, characterization, and application of organic molecules or polymers that show desirable electronic properties such as conductivity. Unlike conventional inorganic conductors and semiconductors, organic electronic materials are constructed from organic (carbon-based) molecules or polymers using synthetic strategies developed in the context of organic chemistry and polymer chemistry.

A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.

In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933.
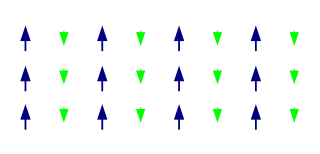
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude, so a spontaneous magnetization remains. This can for example occur when the populations consist of different atoms or ions (such as Fe2+ and Fe3+).

Ferrofluid is a liquid that is attracted to the poles of a magnet. It is a colloidal liquid made of nanoscale ferromagnetic or ferrimagnetic particles suspended in a carrier fluid. Each magnetic particle is thoroughly coated with a surfactant to inhibit clumping. Large ferromagnetic particles can be ripped out of the homogeneous colloidal mixture, forming a separate clump of magnetic dust when exposed to strong magnetic fields. The magnetic attraction of tiny nanoparticles is weak enough that the surfactant's Van der Waals force is sufficient to prevent magnetic clumping or agglomeration. Ferrofluids usually do not retain magnetization in the absence of an externally applied field and thus are often classified as "superparamagnets" rather than ferromagnets.

Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The main advantage of conductive polymers is that they are easy to process, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.

Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one of the most studied conducting polymers.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.

In chemistry, charge-transfer (CT) complex, or electron donor-acceptor complex, describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, i.e., one has at least partial negative charge and the partner has partial positive charge, referred to respectively as the electron acceptor and electron donor. In some cases, the degree of charge transfer is "complete", such that the CT complex can be classified as a salt. In other cases, the charge-transfer association is weak, and the interaction can be disrupted easily by polar solvents.

Alan Graham MacDiarmid, ONZ FRS was a New Zealand-born American chemist, and one of three recipients of the Nobel Prize for Chemistry in 2000.

Sir Richard Henry Friend is a British physicist who was the Cavendish Professor of Physics at the University of Cambridge from 1995 until 2020 and is Tan Chin Tuan Centennial Professor at the National University of Singapore. Friend's research concerns the physics and engineering of carbon-based semiconductors. He also serves as Chairman of the Scientific Advisory Board of the National Research Foundation (NRF) of Singapore.
A single-molecule magnet (SMM) is a metal-organic compound that has superparamagnetic behavior below a certain blocking temperature at the molecular scale. In this temperature range, an SMM exhibits magnetic hysteresis of purely molecular origin. In contrast to conventional bulk magnets and molecule-based magnets, collective long-range magnetic ordering of magnetic moments is not necessary.

A ferrite is an iron oxide-containing magnetic ceramic material. They are ferrimagnetic, meaning they are attracted by magnetic fields and can be magnetized to become permanent magnets. Unlike many ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name of German mining engineer and chemist Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, topological band structure and are actively studied as thermoelectric materials. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)
Molecule-based magnets (MBMs) or molecular magnets are a class of materials capable of displaying ferromagnetism and other more complex magnetic phenomena. This class expands the materials properties typically associated with magnets to include low density, transparency, electrical insulation, and low-temperature fabrication, as well as combine magnetic ordering with other properties such as photoresponsiveness. Essentially all of the common magnetic phenomena associated with conventional transition-metal magnets and rare-earth magnets can be found in molecule-based magnets. Prior to 2011, MBMs were seen to exhibit "magnetic ordering with Curie temperature (Tc) exceeding room temperature".

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.
Lithium hybrid organic batteries are an energy storage device that combines lithium with an organic polymer. For example, polyaniline vanadium (V) oxide (PAni/V2O5) can be incorporated into the nitroxide-polymer lithium iron phosphate battery, PTMA/LiFePO4. Together, they improve the lithium ion intercalation capacity, cycle life, electrochemical performances, and conductivity of batteries.
Kim R. Dunbar is an American inorganic chemist and Distinguished Professor of Chemistry at Texas A&M University. Her research concerns inorganic and coordination chemistry, including molecular magnetism, metals in medicine, supramolecular chemistry Involving anions and anion-pi interactions, and multifunctional materials with organic radicals.
David Collison is a British chemist and a Professor in the Department of Chemistry at The University of Manchester. His research in general is based on inorganic chemistry and magnetochemistry, specifically on coordination chemistry, electron paramagnetic resonance spectroscopy and f-block chemistry.













