
A micelle or micella is an aggregate of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.

In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material.
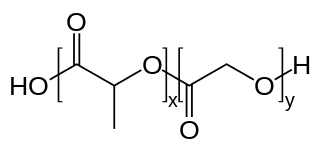
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units, while the approximate length of the propylene glycol block is 56 repeat units. This particular compound is also known by the BASF trade name Pluronic F-127 or by the Croda trade name Synperonic PE/F 127. BASF also offers a pharmaceutical grade, under trade name Kolliphor P 407.
A drug carrier or drug vehicle is a substrate used in the process of drug delivery which serves to improve the selectivity, effectiveness, and/or safety of drug administration. Drug carriers are primarily used to control the release of drugs into systemic circulation. This can be accomplished either by slow release of a particular drug over a long period of time or by triggered release at the drug's target by some stimulus, such as changes in pH, application of heat, and activation by light. Drug carriers are also used to improve the pharmacokinetic properties, specifically the bioavailability, of many drugs with poor water solubility and/or membrane permeability.
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAM, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free-radical polymerization and is readily functionalized making it useful in a variety of applications.
Pluronic P123 is a symmetric triblock copolymer comprising poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO) in an alternating linear fashion, PEO-PPO-PEO. The unique characteristic of PPO block, which is hydrophobic at temperatures above 288 K and is soluble in water at temperatures below 288 K, leads to the formation of micelle consisting of PEO-PPO-PEO triblock copolymers. Some studies report that the hydrophobic core contains PPO block, and a hydrophilic corona consists of PEO block. In 30wt% aqueous solution Pluronic P123 forms a cubic gel phase.

Polylysine refers to several types of lysine homopolymers, which may differ from each other in terms of stereochemistry and link position (α/ε). Of these types, only ε-poly-L-lysine is produced naturally.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
In colloidal chemistry, the critical micelle concentration (CMC) of a surfactant is one of the parameters in the Gibbs free energy of micellization. The concentration at which the monomeric surfactants self-assemble into thermodynamically stable aggregates is the CMC. The Krafft temperature of a surfactant is the lowest temperature required for micellization to take place. There are many parameters that affect the CMC. The interaction between the hydrophilic heads and the hydrophobic tails play a part, as well as the concentration of salt within the solution and surfactants.

A nanocarrier is nanomaterial being used as a transport module for another substance, such as a drug. Commonly used nanocarriers include micelles, polymers, carbon-based materials, liposomes and other substances. Nanocarriers are currently being studied for their use in drug delivery and their unique characteristics demonstrate potential use in chemotherapy. This class of materials was first reported by a team of researchers of University of Évora, Alentejo in early 1960's, and grew exponentially in relevance since then.
The behavior of quantum dots (QDs) in solution and their interaction with other surfaces is of great importance to biological and industrial applications, such as optical displays, animal tagging, anti-counterfeiting dyes and paints, chemical sensing, and fluorescent tagging. However, unmodified quantum dots tend to be hydrophobic, which precludes their use in stable, water-based colloids. Furthermore, because the ratio of surface area to volume in a quantum dot is much higher than for larger particles, the thermodynamic free energy associated with dangling bonds on the surface is sufficient to impede the quantum confinement of excitons. Once solubilized by encapsulation in either a hydrophobic interior micelle or a hydrophilic exterior micelle, the QDs can be successfully introduced into an aqueous medium, in which they form an extended hydrogel network. In this form, quantum dots can be utilized in several applications that benefit from their unique properties, such as medical imaging and thermal destruction of malignant cancers.
Timothy P. Lodge is an American polymer scientist.
Elastin-like polypeptides (ELPs) are synthetic biopolymers with potential applications in the fields of cancer therapy, tissue scaffolding, metal recovery, and protein purification. For cancer therapy, the addition of functional groups to ELPs can enable them to conjugate with cytotoxic drugs. Also, ELPs may be able to function as polymeric scaffolds, which promote tissue regeneration. This capacity of ELPs has been studied particularly in the context of bone growth. ELPs can also be engineered to recognize specific proteins in solution. The ability of ELPs to undergo morphological changes at certain temperatures enables specific proteins that are bound to the ELPs to be separated out from the rest of the solution via experimental techniques such as centrifugation.
Cancer treatments may vary depending on what type of cancer is being targeted, but one challenge remains in all of them: it is incredibly difficult to target without killing good cells. Cancer drugs and therapies all have very low selective toxicity. However, with the help of nanotechnology and RNA silencing, new and better treatments may be on the horizon for certain forms of cancer.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.

Dextran drug delivery systems involve the use of the natural glucose polymer dextran in applications as a prodrug, nanoparticle, microsphere, micelle, and hydrogel drug carrier in the field of targeted and controlled drug delivery. According to several in vitro and animal research studies, dextran carriers reduce off-site toxicity and improve local drug concentration at the target tissue site. This technology has significant implications as a potential strategy for delivering therapeutics to treat cancer, cardiovascular diseases, pulmonary diseases, bone diseases, liver diseases, colonic diseases, infections, and HIV.
Pullulan bioconjugates are systems that use pullulan as a scaffold to attach biological materials to, such as drugs. These systems can be used to enhance the delivery of drugs to specific environments or the mechanism of delivery. These systems can be used in order to deliver drugs in response to stimuli, create a more controlled and sustained release, and provide a more targeted delivery of certain drugs.
Reduction-sensitive nanoparticles (RSNP) consist of nanocarriers that are chemically responsive to reduction. Drug delivery systems using RSNP can be loaded with different drugs that are designed to be released within a concentrated reducing environment, such as the tumor-targeted microenvironment. Reduction-Sensitive Nanoparticles provide an efficient method of targeted drug delivery for the improved controlled release of medication within localized areas of the body.

Alexander Viktorovich Kabanov, is a Russian and American chemist, an educator, an entrepreneur, and a researcher in the fields of drug delivery and nanomedicine.









