
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic.
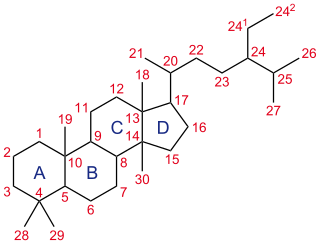
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
In organic chemistry, polyenes are poly-unsaturated, organic compounds that contain at least three alternating double and single carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, resulting in some unusual optical properties. Related to polyenes are dienes, where there are only two alternating double and single bonds.

An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC).

Nystatin, sold under the brandname Mycostatin among others, is an antifungal medication. It is used to treat Candida infections of the skin including diaper rash, thrush, esophageal candidiasis, and vaginal yeast infections. It may also be used to prevent candidiasis in those who are at high risk. Nystatin may be used by mouth, in the vagina, or applied to the skin.

Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. For certain infections it is given with flucytosine. It is typically given intravenously.
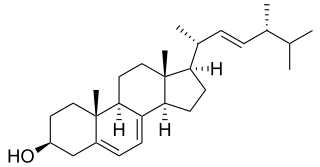
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Natamycin, also known as pimaricin, is an antifungal medication used to treat fungal infections around the eye. This includes infections of the eyelids, conjunctiva, and cornea. It is used as eyedrops. Natamycin is also used in the food industry as a preservative.

Terconazole is an antifungal drug used to treat vaginal yeast infection. It comes as a lotion or a suppository and disrupts the biosynthesis of fats in a yeast cell. It has a relatively broad spectrum compared to azole compounds but not triazole compounds. Testing shows that it is a suitable compound for prophylaxis for those that suffer from chronic vulvovaginal candidiasis.

Filipin is a mixture of chemical compounds first isolated by chemists at the Upjohn company in 1955 from the mycelium and culture filtrates of a previously unknown actinomycete, Streptomyces filipinensis. It was discovered in a soil sample collected in the Philippine Islands, hence the name filipin. The isolate possessed potent antifungal activity. It was identified as a polyene macrolide based on its characteristic UV-Vis and IR spectra.

Hamycin is a pair polyene antimycotic organic compounds described in India. It is a heptaene antifungal compound rather similar in chemical structure to amphotericin B except that it has an additional aromatic group bonded to the molecule. When pure, hamycin is a yellow, powdered solid. There are two versions of hamycin with very similar chemical structures: hamycin A and hamycin B.

Oleandomycin is a macrolide antibiotic. It is synthesized from strains of Streptomyces antibioticus. It is weaker than erythromycin.

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the Cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Perimycin, also known as aminomycin and fungimycin, is polyene antibiotic produced by Streptomyces coelicolor var. aminophilus. The compound exhibits antifungal properties.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.

Dihydromaltophilin, or heat stable anti-fungal factor (HSAF), is a secondary metabolite of Streptomyces sp. and Lysobacter enzymogenes. HSAF is a polycyclic tetramate lactam containing a single tetramic acid unit and a 5,5,6-tricyclic system. HSAF has been shown to have anti-fungal activity mediated through the disruption of the biosynthesis of Sphingolipid's by targeting a ceramide synthase unique to fungi.
Tylactone synthase or TYLS is a Type 1 polyketide synthase. TYLS is found in strains of Streptomyces fradiae and responsible for the synthesis of the macrolide ring, tylactone, the precursor of an antibiotic, tylosin. TYLS is composed of five large multi-functional proteins, TylGI-V. Each protein contains either one or two modules. Each module consists of a minimum of a Ketosynthase (KS), an Acyltransferase (AT), and an Acyl carrier protein (ACP) but may also contain a Ketoreductase (KR), Dehydrotase (DH), and Enoyl Reductase (ER) for additional reduction reactions. The domains of TYLS have similar activity domains to those found in other Type I polyketide synthase such as 6-Deoxyerythronolide B synthase (DEBS). The TYLS system also contains a loading module consisting of a ketosynthase‐like decarboxylase domain, an acyltransferase, and acyl carrier protein. The terminal Thioesterase terminates tylactone synthesis by cyclizing the macrolide ring. After the TYLS completes tylactone synthesis, the tylactone molecule is modified by oxidation at C-20 and C-23 and glycosylation of mycaminose, mycinose, and mycarose to produce tylosin.
Topical antifungaldrugs are used to treat fungal infections on the skin, scalp, nails, vagina or inside the mouth. These medications come as creams, gels, lotions, ointments, powders, shampoos, tinctures and sprays. Most antifungal drugs induce fungal cell death by destroying the cell wall of the fungus. These drugs inhibit the production of ergosterol, which is a fundamental component of the fungal cell membrane and wall.

















