Related Research Articles

The mineral olivine is a magnesium iron silicate with the chemical formula (Mg,Fe)2SiO4. It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers quickly on the surface. For this reason, olivine has been proposed as a good candidate for accelerated weathering to sequester carbon dioxide from the Earth's oceans and atmosphere, as part of climate change mitigation. Olivine also has many other historical uses, such as the gemstone peridot, as well as industrial applications like metalworking processes.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Periclase is a magnesium mineral that occurs naturally in contact metamorphic rocks and is a major component of most basic refractory bricks. It is a cubic form of magnesium oxide (MgO). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite.
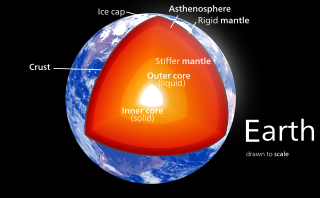
Earth's mantle is a layer of silicate rock between the crust and the outer core. It has a mass of 4.01×1024 kg (8.84×1024 lb) and thus makes up 67% of the mass of Earth. It has a thickness of 2,900 kilometers (1,800 mi) making up about 46% of Earth's radius and 84% of Earth's volume. It is predominantly solid but, on geologic time scales, it behaves as a viscous fluid, sometimes described as having the consistency of caramel. Partial melting of the mantle at mid-ocean ridges produces oceanic crust, and partial melting of the mantle at subduction zones produces continental crust.

The internal structure of Earth is the layers of the Earth, excluding its atmosphere and hydrosphere. The structure consists of an outer silicate solid crust, a highly viscous asthenosphere and solid mantle, a liquid outer core whose flow generates the Earth's magnetic field, and a solid inner core.

The core–mantle boundary (CMB) of Earth lies between the planet's silicate mantle and its liquid iron–nickel outer core, at a depth of 2,891 km (1,796 mi) below Earth's surface. The boundary is observed via the discontinuity in seismic wave velocities at that depth due to the differences between the acoustic impedances of the solid mantle and the molten outer core. P-wave velocities are much slower in the outer core than in the deep mantle while S-waves do not exist at all in the liquid portion of the core. Recent evidence suggests a distinct boundary layer directly above the CMB possibly made of a novel phase of the basic perovskite mineralogy of the deep mantle named post-perovskite. Seismic tomography studies have shown significant irregularities within the boundary zone and appear to be dominated by the African and Pacific Large low-shear-velocity provinces (LLSVP).
A multi-anvil press, or anvil press is a type of device related to a machine press that is used to create extraordinarily high pressures within a small volume.
The transition zone is part of the Earth's mantle, and is located between the lower mantle and the upper mantle, between a depth of 410 and 660 km. The Earth's mantle, including the transition zone, consists primarily of peridotite, an ultramafic igneous rock.

Ringwoodite is a high-pressure phase of Mg2SiO4 (magnesium silicate) formed at high temperatures and pressures of the Earth's mantle between 525 and 660 km (326 and 410 mi) depth. It may also contain iron and hydrogen. It is polymorphous with the olivine phase forsterite (a magnesium iron silicate).
Pyrolite is a term used to characterize a model composition of the Earth's mantle. This model is based on that a pyrolite source can produce the Mid-Ocean Ridge Basalt by partial melting. It was first proposed by Ted Ringwood (1962) as being 1 part basalt and 4 parts harzburgite, but later was revised to being 1 part tholeiitic basalt and 3 parts dunite. The term is derived from the mineral names PYR-oxene and OL-ivine. However, whether pyrolite is representative of the Earth's mantle remains debated.
Ferropericlase or magnesiowüstite is a magnesium/iron oxide with the chemical formula (Mg,Fe)O that is interpreted to be one of the main constituents of the Earth's lower mantle together with the silicate perovskite, a magnesium/iron silicate with a perovskite structure. Ferropericlase has been found as inclusions in a few natural diamonds. An unusually high iron content in one suite of diamonds has been associated with an origin from the lowermost mantle. Discrete ultralow-velocity zones in the deepest parts of the mantle, near the Earth's core, are thought to be blobs of ferropericlase, as seismic waves are significantly slowed as they pass through them, and ferropericlase is known to have this effect at the high pressures and temperatures found deep within the Earth's mantle. In May 2018, ferropericlase was shown to be anisotropic in specific ways in the high pressures of the lower mantle, and these anisotropies may help seismologists and geologists to confirm whether those ultra-low velocity zones are indeed ferropericlase, by passing seismic waves through them from various different directions and observing the exact amount of change in the velocity of those waves.
Akimotoite is a rare silicate mineral in the ilmenite group of minerals, with the chemical formula (Mg,Fe)SiO3. It is polymorphous with pyroxene and with bridgmanite, a natural silicate perovskite that is the most abundant mineral in Earth's silicate mantle. Akimotoite has a vitreous luster, is colorless, and has a white or colorless streak. It crystallizes in the trigonal crystal system in space group R3. It is the silicon analogue of geikielite (MgTiO3).

In chemistry, germanate is a compound containing an oxyanion of germanium. In the naming of inorganic compounds it is a suffix that indicates a polyatomic anion with a central germanium atom, for example potassium hexafluorogermanate, K2GeF6.
Silicate perovskite is either (Mg,Fe)SiO3 or CaSiO3 when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km depth. They are thought to form the main mineral phases, together with ferropericlase.

Large low-shear-velocity provinces (LLSVPs), also called large low-velocity provinces (LLVPs) or superplumes, are characteristic structures of parts of the lowermost mantle, the region surrounding the outer core deep inside the Earth. These provinces are characterized by slow shear wave velocities and were discovered by seismic tomography of deep Earth. There are two main provinces: the African LLSVP and the Pacific LLSVP, both extending laterally for thousands of kilometers and possibly up to 1,000 kilometres vertically from the core–mantle boundary. These have been named Tuzo and Jason respectively, after Tuzo Wilson and W. Jason Morgan, two geologists acclaimed in the field of plate tectonics. The Pacific LLSVP is 3,000 kilometers across and underlies four hotspots on Earth's crust that suggest multiple mantle plumes underneath. These zones represent around 8% of the volume of the mantle, or 6% of the entire Earth.

Patrick Cordier is a mineralogist who uses experimental and numerical approaches to study the plasticity of geological materials. He has authored or co-authored over 200 articles in international scientific journals. He received the Dana Medal from the Mineralogical Society of America in 2016, and is currently a chief editor of the European Journal of Mineralogy. and a member ofInstitut Universitaire de France.
Kepler-277b is the second most massive and third-largest rocky planet ever discovered, with a mass close to that of Saturn. Discovered in 2014 by the Kepler Space Telescope, Kepler-277b is a sub-Neptune sized exoplanet with a very high mass and density for an object of its radius, suggesting a composition made mainly of rock and iron. Along with its sister planet, Kepler-277c, the planet's mass was determined using transit-timing variations (TTVs).

Mantle oxidation state (redox state) applies the concept of oxidation state in chemistry to the study of the Earth's mantle. The chemical concept of oxidation state mainly refers to the valence state of one element, while mantle oxidation state provides the degree of decreasing of increasing valence states of all polyvalent elements in mantle materials confined in a closed system. The mantle oxidation state is controlled by oxygen fugacity and can be benchmarked by specific groups of redox buffers.

The lower mantle, historically also known as the mesosphere, represents approximately 56% of Earth's total volume, and is the region from 660 to 2900 km below Earth's surface; between the transition zone and the outer core. The preliminary reference Earth model (PREM) separates the lower mantle into three sections, the uppermost (660–770 km), mid-lower mantle (770–2700 km), and the D layer (2700–2900 km). Pressure and temperature in the lower mantle range from 24–127 GPa and 1900–2600 K. It has been proposed that the composition of the lower mantle is pyrolitic, containing three major phases of bridgmanite, ferropericlase, and calcium-silicate perovskite. The high pressure in the lower mantle has been shown to induce a spin transition of iron-bearing bridgmanite and ferropericlase, which may affect both mantle plume dynamics and lower mantle chemistry.
The upper mantle of Earth is a very thick layer of rock inside the planet, which begins just beneath the crust and ends at the top of the lower mantle at 670 km (420 mi). Temperatures range from approximately 500 K at the upper boundary with the crust to approximately 1,200 K at the boundary with the lower mantle. Upper mantle material that has come up onto the surface comprises about 55% olivine, 35% pyroxene, and 5 to 10% of calcium oxide and aluminum oxide minerals such as plagioclase, spinel, or garnet, depending upon depth.
References
- ↑ WR Peltier (2007). "Mantle dynamics and the D-doubleprime layer implications of the post-perovskite phase". In Kei Hirose; John Brodholt; Thome Lay; David Yuen (eds.). Post-Perovskite: The Last Mantle Phase Transition (PDF). American Geophysical Union. pp. 217–227. ISBN 978-0-87590-439-9.
- ↑ {{cite journal Ca Ir O3 - Calcium Iridium Oxide3. In san-serif fonts it looks like CalrO3. But this contains Iridium. | doi = 10.1126/science.1095932 | pmid = 15073323 | title = Post-Perovskite Phase Transition in MgSiO3 | first5 = Y | last5 = Ohishi | first4 = N | last4 = Sata | first3 = K | last3 = Kawamura | first2 = K | year = 2004 | last2 = Hirose | last1 = Murakami | first1 = M. | journal = Science | volume = 304 | issue = 5672 | pages = 855–8|bibcode = 2004Sci...304..855M | s2cid = 8616328 }}
- ↑ Oganov, Artem R.; Ono, Shigeaki (2004). "Theoretical and experimental evidence for a post-perovskite phase of MgSiO3 in Earth's D" layer". Nature. 430 (6998): 445–8. arXiv: 0911.3184 . Bibcode:2004Natur.430..445O. doi:10.1038/nature02701. PMID 15269766. S2CID 4418049.
- ↑ Iitaka, T.; Hirose, K.; Kawamura, K.; Murakami, M. (2004). "The elasticity of the MgSiO3 post-perovskite phase in the Earth's lowermost mantle" (PDF). Nature. 430 (6998): 442–5. Bibcode:2004Natur.430..442I. doi:10.1038/nature02702. PMID 15269765. S2CID 4319162.
- ↑ Tsuchiya, Taku; Tsuchiya, Jun; Umemoto, Koichiro; Wentzcovitch, Renata M. (2004). "Phase transition in MgSiO3 perovskite in the earth's lower mantle". Earth and Planetary Science Letters. 224 (3–4): 241. Bibcode:2004E&PSL.224..241T. doi:10.1016/j.epsl.2004.05.017.