Related Research Articles
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases.

In biology, translation is the process in living cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression.
The signal recognition particle (SRP) is an abundant, cytosolic, universally conserved ribonucleoprotein that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes.
Bacterial translation is the process by which messenger RNA is translated into proteins in bacteria.
Eukaryotic translation is the biological process by which messenger RNA is translated into proteins in eukaryotes. It consists of four phases: initiation, elongation, termination, and recapping.
Initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis.
A release factor is a protein that allows for the termination of translation by recognizing the termination codon or stop codon in an mRNA sequence. They are named so because they release new peptides from the ribosome.

Elongation factors are a set of proteins that function at the ribosome, during protein synthesis, to facilitate translational elongation from the formation of the first to the last peptide bond of a growing polypeptide. Most common elongation factors in prokaryotes are EF-Tu, EF-Ts, EF-G. Bacteria and eukaryotes use elongation factors that are largely homologous to each other, but with distinct structures and different research nomenclatures.

EF-Tu is a prokaryotic elongation factor responsible for catalyzing the binding of an aminoacyl-tRNA (aa-tRNA) to the ribosome. It is a G-protein, and facilitates the selection and binding of an aa-tRNA to the A-site of the ribosome. As a reflection of its crucial role in translation, EF-Tu is one of the most abundant and highly conserved proteins in prokaryotes. It is found in eukaryotic mitochondria as TUFM.
Eukaryotic initiation factors (eIFs) are proteins or protein complexes involved in the initiation phase of eukaryotic translation. These proteins help stabilize the formation of ribosomal preinitiation complexes around the start codon and are an important input for post-transcription gene regulation. Several initiation factors form a complex with the small 40S ribosomal subunit and Met-tRNAiMet called the 43S preinitiation complex. Additional factors of the eIF4F complex recruit the 43S PIC to the five-prime cap structure of the mRNA, from which the 43S particle scans 5'-->3' along the mRNA to reach an AUG start codon. Recognition of the start codon by the Met-tRNAiMet promotes gated phosphate and eIF1 release to form the 48S preinitiation complex, followed by large 60S ribosomal subunit recruitment to form the 80S ribosome. There exist many more eukaryotic initiation factors than prokaryotic initiation factors, reflecting the greater biological complexity of eukaryotic translation. There are at least twelve eukaryotic initiation factors, composed of many more polypeptides, and these are described below.
Elongation factor 4 (EF-4) is an elongation factor that is thought to back-translocate on the ribosome during the translation of RNA to proteins. It is found near-universally in bacteria and in eukaryotic endosymbiotic organelles including the mitochondria and the plastid. Responsible for proofreading during protein synthesis, EF-4 is a recent addition to the nomenclature of bacterial elongation factors.

EF-G is a prokaryotic elongation factor involved in protein translation. As a GTPase, EF-G catalyzes the movement (translocation) of transfer RNA (tRNA) and messenger RNA (mRNA) through the ribosome.
Eukaryotic Initiation Factor 2 (eIF2) is an eukaryotic initiation factor. It is required for most forms of eukaryotic translation initiation. eIF2 mediates the binding of tRNAiMet to the ribosome in a GTP-dependent manner. eIF2 is a heterotrimer consisting of an alpha, a beta, and a gamma subunit.
EF-Ts is one of the prokaryotic elongation factors. It is found in human mitochondria as TSFM. It is similar to eukaryotic EF-1B.
Translational regulation refers to the control of the levels of protein synthesized from its mRNA. This regulation is vastly important to the cellular response to stressors, growth cues, and differentiation. In comparison to transcriptional regulation, it results in much more immediate cellular adjustment through direct regulation of protein concentration. The corresponding mechanisms are primarily targeted on the control of ribosome recruitment on the initiation codon, but can also involve modulation of peptide elongation, termination of protein synthesis, or ribosome biogenesis. While these general concepts are widely conserved, some of the finer details in this sort of regulation have been proven to differ between prokaryotic and eukaryotic organisms.
The P-site is the second binding site for tRNA in the ribosome. The other two sites are the A-site (aminoacyl), which is the first binding site in the ribosome, and the E-site (exit), the third. During protein translation, the P-site holds the tRNA which is linked to the growing polypeptide chain. When a stop codon is reached, the peptidyl-tRNA bond of the tRNA located in the P-site is cleaved releasing the newly synthesized protein. During the translocation step of the elongation phase, the mRNA is advanced by one codon, coupled to movement of the tRNAs from the ribosomal A to P and P to E sites, catalyzed by elongation factor EF-G.

EF-P is an essential protein that in bacteria stimulates the formation of the first peptide bonds in protein synthesis. Studies show that EF-P prevents ribosomes from stalling during the synthesis of proteins containing consecutive prolines. EF-P binds to a site located between the binding site for the peptidyl tRNA and the exiting tRNA. It spans both ribosomal subunits with its amino-terminal domain positioned adjacent to the aminoacyl acceptor stem and its carboxyl-terminal domain positioned next to the anticodon stem-loop of the P site-bound initiator tRNA. The EF-P protein shape and size is very similar to a tRNA and interacts with the ribosome via the exit “E” site on the 30S subunit and the peptidyl-transferase center (PTC) of the 50S subunit. EF-P is a translation aspect of an unknown function, therefore It probably functions indirectly by altering the affinity of the ribosome for aminoacyl-tRNA, thus increasing their reactivity as acceptors for peptidyl transferase.
In molecular biology, VAR1 protein domain, otherwise known as variant protein 1, is a ribosomal protein that forms part of the small ribosomal subunit in yeast mitochondria. Mitochondria possess their own ribosomes responsible for the synthesis of a small number of proteins encoded by the mitochondrial genome. VAR1 is the only protein in the yeast mitochondrial ribosome to be encoded in the mitochondria - the remaining approximately 80 ribosomal proteins are encoded in the nucleus. VAR1 along with 15S rRNA are necessary for the formation of mature 37S subunits.
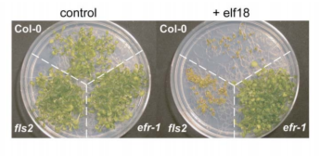
EF-Tu receptor, abbreviated as EFR, is a pattern-recognition receptor (PRR) that binds to the prokaryotic protein EF-Tu in Arabidopsis thaliana. This receptor is an important part of the plant immune system as it allows the plant cells to recognize and bind to EF-Tu, preventing genetic transformation and protein synthesis in pathogens such as Agrobacterium.
Yoshito Kaziro was a Japanese biochemical and medical scientist who performed research on the effects and mechanisms of ATP and GTP driven conformational changes in enzymes and intracellular signaling pathways for over 50 years. He is well-known for his research on various signal transduction pathways involving GTP-binding proteins and the mechanism for biotin dependent carboxylation reactions of Coenzyme A (CoA) proteins.
References
- ↑ Kurzchalia TV, Bommer UA, Babkina GT, Karpova GG (October 1984). "GTP interacts with the gamma-subunit of eukaryotic initiation factor eIF-2". FEBS Letters. 175 (2): 313–6. doi: 10.1016/0014-5793(84)80758-9 . PMID 6566615.
- ↑ Kisselev LL (1995). "Termination of translation in eukaryotes". Biochemistry and Cell Biology. 73 (11–12): 1079–86. doi:10.1139/o95-116. PMID 8722024.
- ↑ Rodnina MV, Savelsbergh A, Katunin VI, Wintermeyer W (January 1997). "Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome". Nature. 385 (6611): 37–41. doi:10.1038/385037a0. PMID 8985244.
- ↑ Freistroffer DV, Pavlov MY, MacDougall J, Buckingham RH, Ehrenberg M (July 1997). "Release factor RF3 in E.coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner". The EMBO Journal. 16 (13): 4126–33. doi:10.1093/emboj/16.13.4126. PMC 1170035 . PMID 9233821.
- ↑ Krab IM, Parmeggiani A (November 1998). "EF-Tu, a GTPase odyssey". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1443 (1–2): 1–22. doi:10.1016/s0167-4781(98)00169-9. PMID 9838020.