Related Research Articles

The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessels. The process is particularly important in controlling hemorrhage and reducing acute blood loss. When blood vessels constrict, the flow of blood is restricted or decreased, thus retaining body heat or increasing vascular resistance. This makes the skin turn paler because less blood reaches the surface, reducing the radiation of heat. On a larger level, vasoconstriction is one mechanism by which the body regulates and maintains mean arterial pressure.
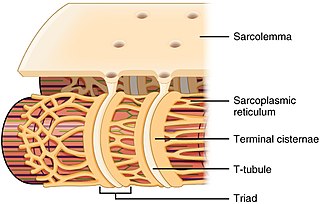
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

Phospholamban, also known as PLN or PLB, is a micropeptide protein that in humans is encoded by the PLN gene. Phospholamban is a 52-amino acid integral membrane protein that regulates the calcium (Ca2+) pump in cardiac muscle cells.
Myocardial contractility represents the innate ability of the heart muscle (cardiac muscle or myocardium) to contract. It is the maximum attainable value for the force of contraction of a given heart. The ability to produce changes in force during contraction result from incremental degrees of binding between different types of tissue, that is, between filaments of myosin (thick) and actin (thin) tissue. The degree of binding depends upon the concentration of calcium ions in the cell. Within an in vivo intact heart, the action/response of the sympathetic nervous system is driven by precisely timed releases of a catecholamine, which is a process that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. The factors causing an increase in contractility work by causing an increase in intracellular calcium ions (Ca++) during contraction.
Calcium-induced calcium release (CICR) describes a biological process whereby calcium is able to activate calcium release from intracellular Ca2+ stores (e.g., endoplasmic reticulum or sarcoplasmic reticulum). Although CICR was first proposed for skeletal muscle in the 1970s, it is now known that CICR is unlikely to be the primary mechanism for activating SR calcium release. Instead, CICR is thought to be crucial for excitation-contraction coupling in cardiac muscle. It is now obvious that CICR is a widely occurring cellular signaling process present even in many non-muscle cells, such as in the insulin-secreting pancreatic beta cells, epithelium, and many other cells. Since CICR is a positive-feedback system, it has been of great interest to elucidate the mechanism(s) responsible for its termination.
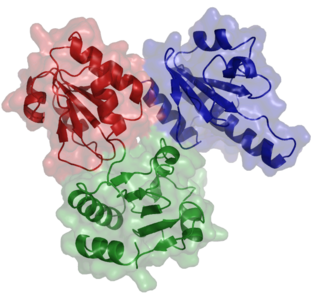
Calsequestrin is a calcium-binding protein that acts as a calcium buffer within the sarcoplasmic reticulum. The protein helps hold calcium in the cisterna of the sarcoplasmic reticulum after a muscle contraction, even though the concentration of calcium in the sarcoplasmic reticulum is much higher than in the cytosol. It also helps the sarcoplasmic reticulum store an extraordinarily high amount of calcium ions. Each molecule of calsequestrin can bind 18 to 50 Ca2+ ions. Sequence analysis has suggested that calcium is not bound in distinct pockets via EF-hand motifs, but rather via presentation of a charged protein surface. Two forms of calsequestrin have been identified. The cardiac form Calsequestrin-2 (CASQ2) is present in cardiac and slow skeletal muscle and the fast skeletal form Calsequestrin-1(CASQ1) is found in fast skeletal muscle. The release of calsequestrin-bound calcium (through a calcium release channel) triggers muscle contraction. The active protein is not highly structured, more than 50% of it adopting a random coil conformation. When calcium binds there is a structural change whereby the alpha-helical content of the protein increases from 3 to 11%. Both forms of calsequestrin are phosphorylated by casein kinase 2, but the cardiac form is phosphorylated more rapidly and to a higher degree. Calsequestrin is also secreted in the gut where it deprives bacteria of calcium ions..

Ca2+ ATPase is a form of P-ATPase that transfers calcium after a muscle has contracted. The two kinds of calcium ATPase are:

The plasma membrane Ca2+ ATPase (PMCA) is a transport protein in the plasma membrane of cells that functions as a calcium pump to remove calcium (Ca2+) from the cell. PMCA function is vital for regulating the amount of Ca2+ within all eukaryotic cells. There is a very large transmembrane electrochemical gradient of Ca2+ driving the entry of the ion into cells, yet it is very important that they maintain low concentrations of Ca2+ for proper cell signalling. Thus, it is necessary for cells to employ ion pumps to remove the Ca2+. The PMCA and the sodium calcium exchanger (NCX) are together the main regulators of intracellular Ca2+ concentrations. Since it transports Ca2+ into the extracellular space, the PMCA is also an important regulator of the calcium concentration in the extracellular space.
The Bowditch effect, also known as the Treppe phenomenon or Treppe effect or Staircase Phenomenon, is an autoregulation method by which myocardial tension increases with an increase in heart rate. It was first observed by Henry Pickering Bowditch in 1871.
ATP2A2 also known as sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) is an ATPase associated with Darier's disease and Acrokeratosis verruciformis.
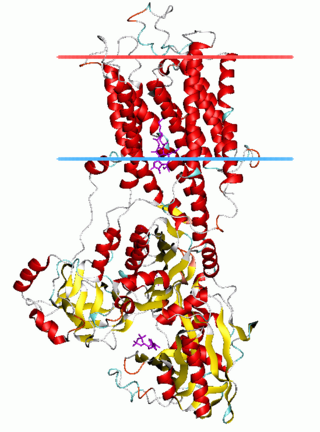
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.
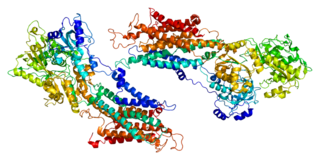
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 (SERCA1) also known as Calcium pump 1, is an enzyme that in humans is encoded by the ATP2A1 gene.

Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 is an enzyme that in humans is encoded by the ATP2A3 gene.

Sarcolipin is a micropeptide protein that in humans is encoded by the SLN gene.
Calcium pumps are a family of ion transporters found in the cell membrane of all animal cells. They are responsible for the active transport of calcium out of the cell for the maintenance of the steep Ca2+ electrochemical gradient across the cell membrane. Calcium pumps play a crucial role in proper cell signalling by keeping the intracellular calcium concentration roughly 10,000 times lower than the extracellular concentration. Failure to do so is one cause of muscle cramps.
CXL 1020 is an experimental drug that is being investigated as a treatment for acute decompensated heart failure. CXL 1020 functions as a nitroxyl donor; nitroxyl is the reduced, protonated version of nitric oxide. Nitroxyl is capable of enhancing left ventricular contractility without increasing heart rate by modifying normal Ca2+ cycling through the sarcoplasmic reticulum as well as increasing the sensitivity of cardiac myofilaments to Ca2+.

Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
References
- 1 2 3 4 Marín-García, José (2014-01-01), Marín-García, José (ed.), "Chapter 23 - Gene- and Cell-Based Therapy for Cardiovascular Disease", Post-Genomic Cardiology (Second Edition), Boston: Academic Press, pp. 783–833, doi:10.1016/b978-0-12-404599-6.00023-8, ISBN 978-0-12-404599-6 , retrieved 2020-12-28
- ↑ de Meis L; Oliveira GM; Arruda AP; Santos R; Costa RM; Benchimol M (2005). "The thermogenic activity of rat brown adipose tissue and rabbit white muscle Ca2+-ATPase". IUBMB Life. 57 (4–5): 337–45. doi: 10.1080/15216540500092534 . PMID 16036618.
- ↑ Arruda AP; Nigro M; Oliveira GM; de Meis L (June 2007). "Thermogenic activity of Ca2+-ATPase from skeletal muscle heavy sarcoplasmic reticulum: the role of ryanodine Ca2+ channel". Biochim. Biophys. Acta. 1768 (6): 1498–505. doi: 10.1016/j.bbamem.2007.03.016 . PMID 17466935.
- ↑ Bal, Naresh C.; Periasamy, Muthu (2020-03-02). "Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis". Philosophical Transactions of the Royal Society B: Biological Sciences. 375 (1793): 20190135. doi:10.1098/rstb.2019.0135. PMC 7017432 . PMID 31928193.
- ↑ Legendre, Lucas J.; Davesne, Donald (2020-03-02). "The evolution of mechanisms involved in vertebrate endothermy". Philosophical Transactions of the Royal Society B: Biological Sciences. 375 (1793): 20190136. doi: 10.1098/rstb.2019.0136 . PMC 7017440 . PMID 31928191.
- ↑ MacLennan, David H.; Kranias, Evangelia G. (July 2003). "Phospholamban: a crucial regulator of cardiac contractility". Nature Reviews Molecular Cell Biology. 4 (7): 566–577. doi:10.1038/nrm1151. PMID 12838339. S2CID 3050392.
- ↑ Gonnot, Fabrice; Boulogne, Laura; Brun, Camille; Dia, Maya; Gouriou, Yves; Bidaux, Gabriel; Chouabe, Christophe; Crola Da Silva, Claire; Ducreux, Sylvie; Pillot, Bruno; Kaczmarczyk, Andrea; Leon, Christelle; Chanon, Stephanie; Perret, Coralie; Sciandra, Franck (2023-06-08). "SERCA2 phosphorylation at serine 663 is a key regulator of Ca2+ homeostasis in heart diseases". Nature Communications. 14 (1): 3346. doi:10.1038/s41467-023-39027-x. ISSN 2041-1723. PMC 10250397 . PMID 37291092.
- ↑ Arruda, AP; Oliveira, GM; Carvalho, DP; De Meis, L (November 2005). "Thyroid hormones differentially regulate the distribution of rabbit skeletal muscle Ca(2+)-ATPase (SERCA) isoforms in light and heavy sarcoplasmic reticulum". Molecular Membrane Biology. 22 (6): 529–37. doi: 10.1080/09687860500412257 . PMID 16373324. S2CID 29949157.
- ↑ Chang, KC; Figueredo, VM; Schreur, JH; Kariya, K; Weiner, MW; Simpson, PC; Camacho, SA (1 October 1997). "Thyroid hormone improves function and Ca2+ handling in pressure overload hypertrophy. Association with increased sarcoplasmic reticulum Ca2+-ATPase and alpha-myosin heavy chain in rat hearts". The Journal of Clinical Investigation. 100 (7): 1742–9. doi:10.1172/JCI119699. PMC 508357 . PMID 9312172.
- ↑ Kaasik, Allen; Minajeva, Ave; Paju, Kalju; Eimre, Margus; Seppet, Enn K. (1997). "Thyroid hormones differentially affect sarcoplasmic reticulum function in rat atria and ventricles". Molecular and Cellular Biochemistry. 176 (1/2): 119–126. doi:10.1023/A:1006887231150. PMID 9406153. S2CID 8199751.
- ↑ Müller, Patrick; Leow, Melvin Khee-Shing; Dietrich, Johannes W. (15 August 2022). "Minor perturbations of thyroid homeostasis and major cardiovascular endpoints—Physiological mechanisms and clinical evidence". Frontiers in Cardiovascular Medicine. 9: 942971. doi: 10.3389/fcvm.2022.942971 . PMC 9420854 . PMID 36046184.